Introduction
The origin of the superficial circumflex iliac artery (SCIA) is from the external iliac artery (EIA) [1], or from the common femoral artery (CFA) [2]. Sometimes it may come as a branch of the superficial femoral artery (SFA), or profound femoral artery (PFA) [3, 4]. After originating, this artery will give 2 branches: superficial branch (SB), and profound branch (PB) [2], the first will be responsible for the antero-lateral abdominal wall blood supply, and the second will descend down to the sartorius muscle [2, 3]. It may have a variable diameter [1], and sometimes perforant branches [3].
A lack of knowledge in surgical anatomy along with the anatomical variability of certain vessels like the deep circumflex iliac artery (DCIA) may lead to severe surgical complications [5]. Skin flap reconstruction surgery is of great interest when it comes to the SCIA [6-8], and it is proven to be reliable in terms of blood supply [2]. Bone grafts for the surgical repair of the defects in the iliac bone and fibula can be regarded as a SCIA flap [9]. The anatomical variability of this artery should be considered in procedures that involve a femoral nerve (FN) block [10], and when predicting lymphedema patterns [11]. Surgical interventions in the groin and thigh regions have implications that regard the anatomical variability of this artery [12].
The anatomical variability of the neighboring structures like the FN, and DCIA may complicate the accurate identification of the SCIA [13, 14]. This necessitates advanced knowledge for the precise determination of these structures.
Vascular compression syndromes like May-Thurner syndrome [15] may co-interest the SCIA in an unexpected way because the arterial circulation in this region may be highly dependent on the vessels like CFA, EIA, and their branches that could in turn lead to the pathological alterations in the ipsilateral lower extremity [16].
The access to the femoral arterial system in the thigh region is made through a transverse incision, or in the groin region with a vertical incision for vascular, and endovascular surgical interventions [17], thus making the SCIA vulnerable to potential lesions in both cases.
The goal of this study was to determine the anatomical variability of the SCIA depending on the origin, gender, and laterality.
Material and methods
Statistical considerations. We retrospectively reviewed 2158 Doppler ultrasonography images of the anterior thigh region from the Republican Medical Diagnostic Center, Functional Diagnosis Department, Chisinau, Republic of Moldova. These images were collected between 01.01.2020-31.12.2022. The randomized selection of the patients was not required, as all available cases were included in the study. The study population consisted of 885 females (41.01%) and 1,273 males (58.99%). We have calculated the mean age of the patients, the absolute, and relative values of the incidence in anatomical variability based on the origin, gender, and laterality.
Inclusion and exclusion criteria. We included only patients that underwent imaging investigations on both legs and excluded duplicate cases (i.e., patients who had multiple serial investigations over time). All included cases were eligible for visualizing the tissues, vessels, and some nerves that were located nearby. No data was available on the antero-lateral abdominal wall, or lower parts of the thigh.
Equipment. The „GE Healthcare Vscan AirTM” ultrasonografic device was used in order to perform these investigations. A single sensory device was of interest, operating in Doppler mode. No electronic applications were used, all the investigations were described manually.
Literature review. We have reviewed the available literature from PubMed, Google Scholar, HINARI, EMBASE, Elsevier, and Research Gate in order to find the most suitable research papers and have completed a comparative review in order to emphasize the significance of our findings. Overall, 28 literary sources were selected. Additionally, we have reviewed a book. A total of 29 bibliographic sources were referenced in this study/
Results
The mean age of the patients was 63.4±10.68 years. In 387 cases (17.93%), the SCIA originated from the CFA, while in 1771 cases (82.07%), it emerged from the EIA. Among the cases withorigin from the CFA,163 (7.55%) were female and 224 (10.38%) were male. For those with origin from the EIA were 722 (33.46%) were female, and 1049 (48.61%) were male. Thus, the most common origin of the SCIA was from the EIA.
Taking into consideration the gender, the SCIA originated from the CFA in 42.12% of female cases and in 57.88% of male cases. For the EIA origin, 40.77% of cases were female, and49.23% were male. Comparing these distributions, we conclude that there are no significant gender differences for the anatomical variability of the origin for this artery.
Based on laterality, the SCIA originated from the CFA in males as follows: 118 (5.5%) cases unilaterally on the left side, 82 (3.78%) cases unilaterally on the right side, and 24 (1.1%) cases bilaterally. In females, the origin from the CFA was observed in 76 (3.52%) cases unilaterally on the left side, 60 (2.78%) cases unilaterally on the right side, and 27 (1.25%) cases bilaterally. In total, there were 194 (8.99%) cases unilaterally on the left side, 142 (6.58%) cases unilaterally on the right side, and 51 (2.36%) cases bilaterally. The most common incidence of origin from the CFA was unilaterally on the left side.
Proportionally, males, the SCIA originated from the CFA unilaterally on the left side in 30.49% of cases, unilaterally on the right side 21.19% of cases on, and bilaterally in 6.2% of cases. In females the corresponding proportions were 19.64% on the left side, 15.50% on the right side, and 6.98% bilaterally. When considering proportions within each gender separately, in males 52.68% of cases were unilateral on the left side, 36.61% were unilateral on the right side, and 10.71% were bilaterally. In females 46.63% were unilateral on the left side, 36.81% were unilateral on the right side, and 16.56% were bilateral. The bilateral origin of the SCIA from the CFA is more common in the females compared to males, while the most frequent pattern in both genders was a unilateral origin on the left side.
Age was not quantified as a factor that may influence the anatomical variability of SCIA. There were no variations that regarded supranumerary arteries, or trunks from which these arteries may emerge. Arterio-venous fistulas along with many other anomalies were considered as a potential bias factor but we have not identified any in our study. Atherosclerosis, thrombosis, thromboembolism, arterial dysplasia, and calcifications were impending factors in some cases. The SCIA had a diameter that may be affected by these conditions and yet remain visible in the Doppler ultrasonography sections.
|
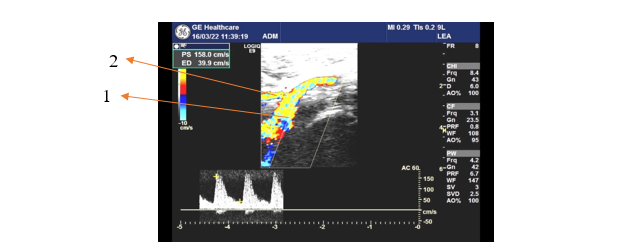
Fig. 1 Left upper thigh anterior Doppler ultrasonography 1 – common femoral artery; 2 – superficial circumflex iliac artery |
Figure 1 shows the ultrasonographic image of a 72-year-old patient with the SCIA originating from the CFA. The emergence was directly from the main arterial trunk without a common origin with another artery, or a second artery of the same kind. The SCIA on the right upper thigh of the same patient had an identical origin. No pathological conditions were detected in this patient.
Discussion
According to Gandolfi et al. (2020), and Song et al. (2020) the most frequent origin of the SCIA was from the CFA [3, 18]. In our study the most common origin was from the EIA, just as in the reports of Kovanov V. V. (1974) [1].
There were no significant gender differences in the proportionality of this artery’s origin while the laterality may play a key role, the bilateral origin of the SCIA from the CFA is more common in females, and in both genders the left unilateral type is more frequent rather than the right unilateral, or bilateral. No previous studies or literature sources were available form comparison with these findings.
This information is of great interest during potential groin, and thigh hernia repairs (direct, and indirect inguinal, or femoral) [12], femoral artery incisions [17], or for flap reconstruction surgical interventions that may involve bone grafting [9].
A study conducted in Denmark [19] has proven that a laparoscopic approach reduces the incidence of recurrences in femoral hernias, and also there is a higher mortality in the emergency femoral hernia repairs [20], thus an origin of the SCIA from the EIA may pose a risk for these interventions, especially due to the fact that it is highly prevalent (82.07%) compared to the origin from the CFA (17.93%).
Flap reconstruction surgeries are essential for revascularizing traumatized regions, and portions of the skin affected by burns [21, 22]. Our findings may be of great interest when identifying the origin of the SCIA in order to apply a ligature, although the SCIA is not involved in the collateral circulation of its anatomical region [23] while the DCIA is involved in this compensatory mechanism [24].
The vertical incision may cause increased morbidity due to an increased incidence of SCIA lesion, the same was stated in a study made by Caiati et al. in 2000 where the oblique incision is proposed for the purpose of reducing this burden [25] while the transverse incision is not proven to reduce the morbidity compared to the vertical one [17].
Other implications like truncal blocks [10], and the prediction of post-surgical complications is also of great interest in this anatomical variability [12], thus this study may provide useful data for further assessment of these patients because the identification of the FN, or DCIA can become even more difficult after we have reported these anatomical variations [13, 14].
Vascular compression syndromes may be of interest in this context, because they may induce alternations in the SCIA via chronic ischemia, and excessive continuous collateral circulation for this particular anatomical region [15, 16]. The higher prevalence of bilateral SCIA origin from the CFA in females may be of particular interest in the May-Thurner syndrome, which predominantly affects women, especially in their second or third decade as a result of multifactorial risk exposure [16, 26]. This syndrome is also treated using endovascular interventions that require further knowledge of the groin, and thigh regional anatomy [27].
There were no literature reports that regarded the aneurysms, or pseudoaneurysms of the SCIA but there were some that were located in the DCIA during diagnostic maneuvers, both iatrogenic, and idiopathic [28, 29], thus we may hypothesize that SCIA could be affected as well due to their similar morphological macroscopic, and microscopic aspect.
We were unable to measure the internal diameter of the SCIA. The trajectory of both superficial and deep branches was not assessed. The visualization of the perforasomes was not feasible. We could not have compared the topography of this artery in relation to other branches in the same anatomical region.
Conclusions
In our study the most common origin of the superficial circumflex iliac artery was from the external iliac artery (EIA). There was no significant difference between genders in the overall origin of the SCIA. However, a bilateral origin from the common femoral artery (CFA) was more frequently observed in females, while the most common pattern in both genders was a unilateral origin on the left side. Age was not assessed as a factor that determined the anatomical variability.
Competing interest
None declared.
Contribution of authors
DC designed the study, collected the data and analyzed it, VN revised the results critically and evaluated their applicability, SV revised the manuscript critically, SC revised the manuscript critically.
Ethics approval
No approval was required for this study.
Funding
Authors’ declare no external funding.
Authors’ ORCID IDs
Dan Croitoru – https://orcid.org/0000-0002-8915-0157
Viorel Nacu – https://orcid.org/0000-0003-2274-9912
Sergiu Vișnevschi – https://orcid.org/0000-0002-0950-1720
Stanislav Coșciug – https://orcid.org/0009-0001-6505-2712
Bibliography
Kovanov VV, Anikina TI. Khirurgicheskaia anatomiia arterii cheloveka [Surgical anatomy of human arteries]. Moscow: Meditsina; 1974. 359 p. Russian.
Zubler C, Haberthür D, Hlushchuk R, Djonov V, Constantinescu M, Olariu R. The anatomical reliability of the superficial circumflex iliac artery perforator (SCIP) flap. Ann Anat. 2021;234:1-9. doi: 10.1016/j.aanat.2020.151624.
Gandolfi S, Postel F, Auquit-Auckbur I, Boissière F, Pelissier P, Casoli V, Duparc F. Vascularization of the superficial circumflex iliac perforator flap (SCIP flap): an anatomical study. Surg Radiol Anat. 2020;42(4):473-481. doi: 10.1007/s00276-019-02402-9.
Yoshimatsu H, Steinbacher J, Meng S, Hamscha U, Weninger W, Tinhofer I, Harima M, Fuse Y, Yamamoto T, Tzou C. Superficial circumflex iliac artery perforator flap: an anatomical study of the correlation of the superficial and the deep branches of the artery and evaluation of perfusion from the deep branch to the sartorius muscle and the iliac bone. Plast Reconstr Surg. 2019;143(2):589-602. doi: 10.1097/PRS.0000000000005282.
Singh B, Gupta V, Gupta S. Pseudoaneurysm: a complication of laparoscopic inguinal hernia repair. Int J Surg Case Rep. 2019;54:39-41. doi: 10.1016/j.ijscr.2018.11.045.
Messa CA 4th, Carney MJ 3rd, Tantillo K, Othman S, Moores C, Mirzabeigi MN, Weissler JM, Cook T, Kovach SJ. Characteristics of the superficial circumflex iliac artery perforator flap in a western population and a practice approach for free flap reconstruction. J Reconstr Microsurg. 2021;37(6):486-491. doi: 10.1055/s-0040-1719051.
Hong JP. The superficial circumflex iliac artery perforator flap in lower extremity reconstruction. Clin Plast Surg. 2021;48(2):225-233. doi: 10.1016/j.cps.2020.12.005.
Berner JE, Nikkhah D, Zhao J, Prousskaia E, Teo TC. The Versatility of the superficial circumflex iliac artery perforator flap: a single surgeon's 16-year experience for limb reconstruction and a systematic review. J Reconstr Microsurg. 2020;36(2):93-103. doi: 10.1055/s-0039-1695051.
Yoshimatsu H, Iida T, Yamamoto T, Hayashi A. Superficial circumflex iliac artery-based iliac bone flap transfer for reconstruction of bony defects. J Reconstr Microsurg. 2018;34(9):719-728. doi: 10.1055/s-0038-1651489.
Ogami K, Murata H, Sakai A, Sato S, Saiki K, Okamoto K, Manabe Y, Hara T, Tsurumoto T. Deep and superficial circumflex iliac arteries and their relationship to the ultrasound-guided femoral nerve block procedure: a cadaver study. Clin Anat. 2017;30(3):413-420. doi: 10.1002/ca.22852.
Campos JL, Suominen S, Pons G, Al-Sakkaf AM, Lusetti IL, Sirota M, Vela FJ, Pires L, Sánchez-Margallo FM, Abellán E, Masiá J. Lymphatic patterns in the superficial circumflex iliac artery perforator flap. J Reconstr Microsurg. 2024 Jul 9. doi: 10.1055/a-2340-9629. Epub ahead of print.
Brunetti B, Camilloni C, Putti A, Petrucci V, Pazzaglia M, Papalia R, Persichetti P. Use of superficial circumflex iliac artery perforator flap for soft tissues and lymphatic genital reconstruction after hidradenitis suppurativa resection. J Plast Reconstr Aesthet Surg. 2024;88:122-124. doi: 10.1016/j.bjps.2023.10.140.
Park JA, Lee SH, Koh KS, Song WC. Femoral nerve split with variant iliacus muscle: a potential source of femoral nerve entrapment. Surg Radiol Anat. 2020;42(10):1255-1257. doi: 10.1007/s00276-020-02502-x.
Sarna K, Amuti T, Butt F, Kamau M, Muriithi A. Variations of the anatomy and bony landmarks of deep circumflex iliac artery in a select Kenyan population. Craniomaxillofac Trauma Reconstr. 2020;13(4):300-304. doi: 10.1177/1943387520958333.
Farina R, Foti PV, Pennisi I, Vasile T, Clemenza M, Rosa G, Crimi L, Catalano M, Vacirca F, Basile A. Vascular compression syndromes: a pictorial review. Ultrasonography. 2022;41(3):444-461. doi: 10.14366/usg.21233.
Poyyamoli S, Mehta P, Cherian M, Anand RR, Patil SB, Kalva S, Salazar G. May-Thurner syndrome. Cardiovasc Diagn Ther. 2021;11(5):1104-1111. doi: 10.21037/cdt.2020.03.07.
Canteras M, Baptista-Silva JC, do Carmo Novaes F, Cacione DG. Transverse versus vertical groin incision for femoral artery approach. Cochrane Database Syst Rev. 2020;4(4):1-32. doi: 10.1002/14651858.CD013153.pub2.
Song C, Bhogesha S, Chu S, Song C. Review of the superficial circumflex iliac artery perforator flap: recommendations to the approach of a groin perforator flap. Australas J Plast Surg. 2020;3(2):20-29. doi: 10.34239/ajops.v3n2.193.
Andresen K, Bisgaard T, Kehlet H, Wara P, Rosenberg J. Reoperation rates for laparoscopic vs open repair of femoral hernias in Denmark: a nationwide analysis. JAMA Surg. 2014;149(8):853-7. doi: 10.1001/jamasurg.2014.177.
Dahlstrand U, Wollert S, Nordin P, Sandblom G, Gunnarsson U. Emergency femoral hernia repair: a study based on a national register. Ann Surg. 2009;249(4):672-6. doi: 10.1097/SLA.0b013e31819ed943.
Hussain T, Khan FH, Rahman OU, Beg MSA. Superficial circumflex iliac artery free flap for coverage of hand injuries. Cureus. 2022;14(11):e31520. doi: 10.7759/cureus.31520.
Prasetyo AT, Hasibuan LY, Arsyad M. Pedicled abdominal skin flap technique for fingers salvaging and reconstruction in a complex palmar hand burn injury: a case report. Int J Surg Case Rep. 2024;114:109199. doi: 10.1016/j.ijscr.2023.109199.
Akinwande O, Ahmad A, Ahmad S, Coldwell D. Review of pelvic collateral pathways in aorto-iliac occlusive disease: demonstration by CT angiography. Acta Radiol. 2015;56(4):419-427. doi: 10.1177/0284185114528172.
Hardman RL, Lopera JE, Cardan RA, Trimmer CK, Josephs SC. Common and rare collateral pathways in aortoiliac occlusive disease: a pictorial essay. Am J Roentgenol. 2011;197(3):519-524. doi: 10.2214/AJR.10.5896.
Caiati JM, Kaplan D, Gitlitz D, Hollier LH, Marin ML. The value of the oblique groin incision for femoral artery access during endovascular procedures. Ann Vasc Surg. 2000;14(3):248-253. doi: 10.1007/s100169910042.
Mousa AY, AbuRahma AF. May-Thurner syndrome: update and review. Ann Vasc Surg. 2013;27(7):984-995. doi: 10.1016/j.avsg.2013.05.001.
Ibrahim W, Al Safran Z, Hasan H, Zeid WA. Endovascular management of may-thurner syndrome. Ann Vasc Dis. 2012;5(2):217-221. doi: 10.3400/avd.cr.12.00007.
Satija B, Kumar S, Duggal RK, Kohli S. Deep circumflex iliac artery pseudoaneurysm as a complication of paracentesis. J Clin Imaging Sci. 2012;2:1-4. doi: 10.4103/2156-7514.94022.
Miyagi K, Mulchandani M, Marks DJB, Mohamed M. Aneurysm of the deep circumflex iliac artery: a rare cause of rectus sheath haematoma. Eur J Vasc Endovasc Surg Extra. 2010;19(2):e19-e21. doi: 10.1016/j.ejvsextra.2009.11.002.