Introduction
Psoriatic arthritis (PsA) is a complex condition, with its pathogenesis involving both genetic and immunological factors. HLA antigens play a crucial role in determining genetic predisposition and influencing the severity and clinical variability of the disease [1]. Studies have shown that certain HLA alleles, such as HLA-B27, B8, B11, and B62, are associated with severe forms of PsA, while others, like HLA-A2, A3, and A5, may have a protective effect [2, 3].
Despite progress in identifying the role of these antigens, their specific relationship with various clinical variants of the disease remains incompletely understood. Investigating HLA antigens in patients with PsA in the context of clinical diversity can provide valuable insights for early diagnosis, risk stratification, and personalized treatment [1, 4, 5]. In the Republic of Moldova, such studies are rare but essential for gaining a deeper understanding of the genetic impact on this pathology and for tailoring therapeutic interventions to the specific needs of the local population.
The aim of the study is to evaluate the diversity of HLA antigens in patients with psoriatic arthritis, depending on the clinical variants of the disease.
Material and methods
To achieve the study's aim and objectives, a cohort of 103 patients diagnosed with PsA according to the CASPAR criteria (2006) was selected. The patients received treatment in the rheumatology and arthrology departments of Timofei Moșneaga Republican Clinical Hospital and Holy Trinity Municipal Clinical Hospital in Chișinău during the period 2005–2024. Inclusion criteria: confirmed diagnosis of PsA according to CASPAR criteria (2006); availability of relevant clinical and laboratory data; absence of other inflammatory rheumatic diseases (such as rheumatoid arthritis or systemic lupus erythematosus); informed consent obtained from patients for participation in the study. The study was approved by the Research Ethics Committee of Nicolae Testemițanu State University of Medicine and Pharmacy (No.21 from 21.12.2019).
To investigate the relationships between the clinical-evolutionary variants of PsA and genetic factors, participants were divided into two groups. Group I: 76 patients with PsA associated with cutaneous psoriasis; group II: 27 patients with PsA without cutaneous manifestations.
To minimize selection bias, patients were allocated into comparable groups based on age, sex, disease duration, and arthritis severity. The demographic and clinical variables distribution showed no statistically significant differences (p > 0.05). The mean age of patients in Groups I and II was comparable (48.7±10.5 vs. 46.9±11.2, p > 0.05), as was the mean PsA duration (7.3±3.1 vs. 7.1±2.7 years, p > 0.05). Disease severity was evaluated using the DAPSA (Disease Activity in Psoriatic Arthritis) score, and patients were stratified into categories of low (<20), moderate (20–50), and high activity (>50).
Patients were further subdivided into five subgroups based on the clinical variant of the disease:
- Group I: oligoarticular – 13 patients; polyarticular – 23 patients; distal interphalangeal – 11 patients; axial – 21 patients; mutilans – 8 patients.
- Group II: oligoarticular – 5 patients; distal interphalangeal – 4 patients; polyarticular –: 9 patients; axial – 5 patients; mutilans – 4 patients.
HLA antigen determination was performed using standard molecular typing techniques via PCR-SSP (polymerase chain reaction with sequence-specific primers). Imaging investigations included joint ultrasound and MRI to identify structural changes.
This structured grouping and the application of rigorous investigative methods enable a detailed analysis of the relationship between HLA antigens and clinical variants of PsA, providing valuable insights for early diagnosis and risk stratification.
The statistical analysis was performed using Microsoft Excel, Statistica 9.0, and EpiInfo version 5. Descriptive statistics were applied, with mean±standard deviation (M±SD) for normally distributed variables and median (Me) with interquartile range (25%; 75%) for non-normal distributions. The Student's T-test was used for parametric data, and the Mann-Whitney U test for non-parametric data. Non-parametric correlations were assessed using Spearman's method. The significance of differences in HLA antigens and disease risk measures was determined using the X² test, Fisher's exact test, and odds ratio (OR), with results considered significant at p<0.05.
Results
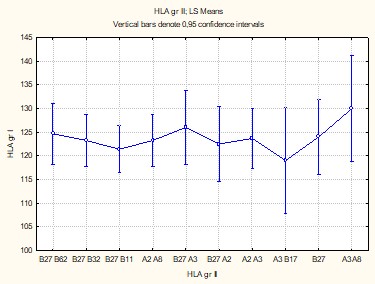
Factorial analysis revealed significant correlations between HLA antigens identified in patients with PsA and cutaneous psoriasis (p = 0.00043), except for HLA-B32 and HLA-B62 antigens (p>0.05). The antigens HLA-B27/A2 (CI+95% = 217.7), HLA-B27 (CI+95% = 187.5), HLA-B27/A3 (CI+95% = 176.8), and HLA-A2/A3 (CI+95% = 140.5) were common to both subgroups, leading to similar osteoarticular impairments without influencing the distinct evolution of the disease (Figure 1).
In the group of patients with PsA associated with cutaneous psoriasis, HLA-A2/A5 (CI+95% = 193.5), HLA-B62/B8 (CI+95% = 211.3), HLA-B27/B8 (CI+95% = 207.1), and HLA-B27/32 (CI+95% = 188.2) antigens were associated with pronounced systemic involvement. Conversely, in patients without cutaneous psoriasis, HLA-B27/62 (CI+95% = 223.6) and HLA-B27/11 (CI+95% = 215.6) antigens showed a high prevalence, reflecting a distinct immunological profile.
These results confirm the presence of specific genetic HLA characteristics in PsA, with variations among clinical subgroups. They underscore the importance of these antigens in determining the disease's severity and systemic manifestations.
|
Fig. 1 Factorial correlation of HLA antigens among patient groups with PsA associated with cutaneous psoriasis (Group I) and without cutaneous psoriasis (Group II), p > 0.05. Note: HLA – human leukocyte antigen; PsA – psoriatic arthritis |
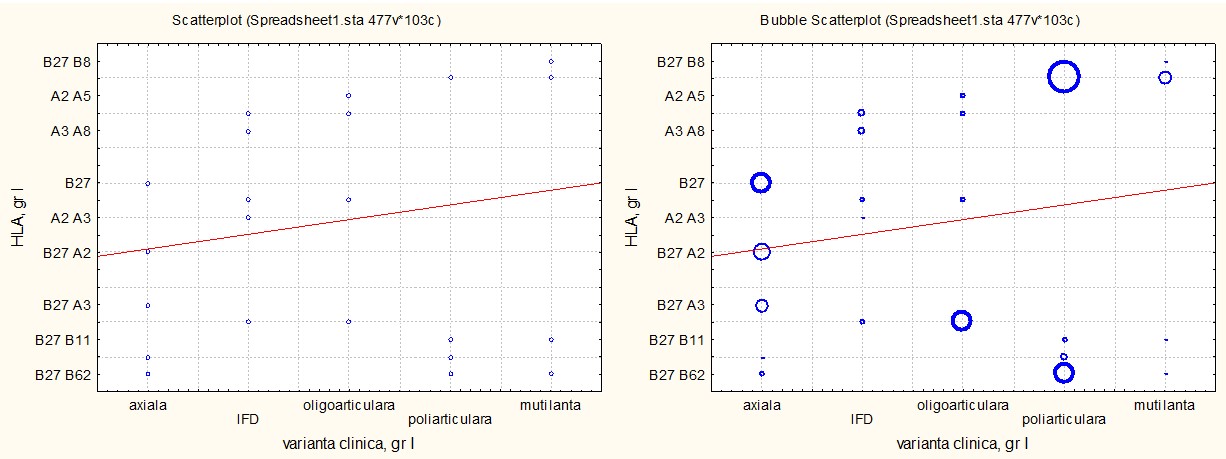
The associative analysis using ANOVA revealed significant correlations between the clinical variants of PsA and HLA antigen sets in patients with cutaneous psoriasis (p<0.01).
For the axial variant, HLA-B27, HLA-B27/A2, and HLA-B27/A3 antigens were predominant, reflecting their involvement in inflammatory mechanisms of the spine and sacroiliac joints. In the distal interphalangeal variant, HLA-A2/A3, HLA-A2/A5, and HLA-A2/A8 antigens were associated with relatively benign involvement of small joints. The oligoarticular variant was correlated with HLA-B27/A3, HLA-A2/A5, and HLA-A3/A8, indicating a slower and less aggressive progression.
The polyarticular variant showed an association with HLA-B27/B8, HLA-B27/B62, and HLA-B27/B11, which were frequently observed in patients with systemic involvement and rapid progression. The most severe form, the mutilans variant, was dominantly associated with HLA-B27/B8, indicating a genetic risk for extensive joint destruction.
These findings underscore the immunogenetic role of HLA antigens in the clinical variability of PsA, offering perspectives for personalized diagnostic and therapeutic strategies. The relationships are detailed in Figure 2, which highlights the antigen-specific differences for each clinical subtype.
|
Fig. 2 The associative correlation of HLA antigens with clinical variants of PsA in patients with PsA associated with cutaneous psoriasis, p < 0.01. Note: HLA – human leukocyte antigen; PsA – psoriatic arthritis |
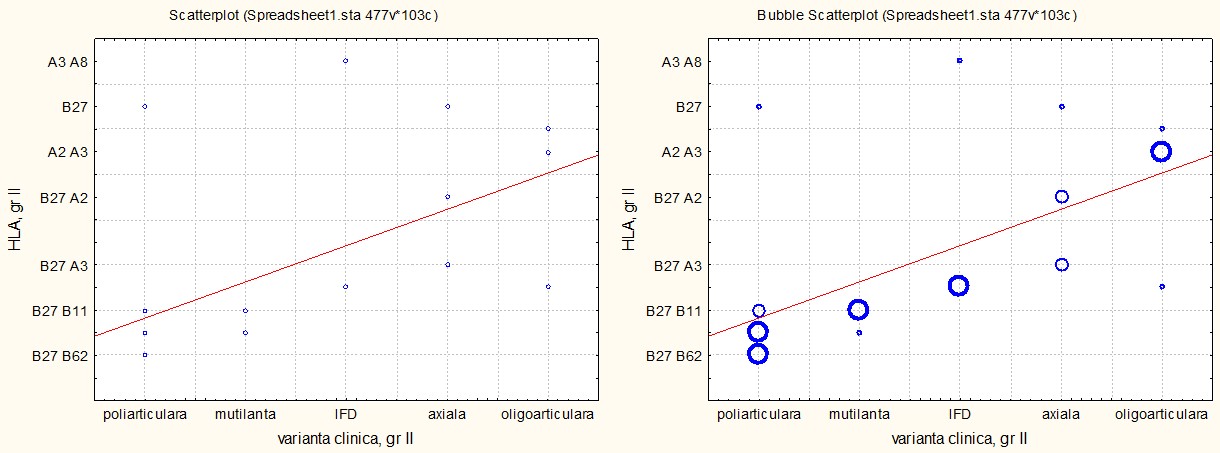
The analysis of HLA antigens in the group of patients with PsA without cutaneous psoriasis revealed a general pattern similar to that observed in the group with cutaneous psoriasis, but with significant differences. The HLA-B27 antigen showed a uniform distribution across all clinical variants, unlike its predominant association with the axial form in the group with skin involvement. This suggests a more generalized genetic role of HLA-B27 in the pathogenesis of PsA without cutaneous manifestations.
Furthermore, an increased similarity was observed between the distal interphalangeal and oligoarticular variants, both associated with HLA-B27/A3 and HLA-A3/A8 alleles (for the distal interphalangeal form) and HLA-B27/A3 and HLA-A2/A3 (for the oligoarticular form). This antigenic overlap indicates common pathogenic mechanisms, despite differing clinical manifestations between these forms.
Figure 3 illustrates the distribution of HLA antigens, highlighting the relatively reduced specificity of HLA-B27 for the axial form and the antigenic similarity between the distal interphalangeal and oligoarticular forms. These observations suggest that the distribution of HLA antigens influences the clinical characteristics of PsA depending on the presence or absence of cutaneous psoriasis.
|
Fig. 3 Associative correlation of HLA antigens with clinical variants of PsA in patients with PsA without cutaneous psoriasis, p < 0.01. Note: HLA – human leukocyte antigen; PsA – psoriatic arthritis |
The association of HLA antigens between the polyarticular and mutilans variants of PsA suggests a direct evolutionary link between these forms. In both studied groups, HLA-B27/B8, HLA-B27/B62, and HLA-B27/B11 were strongly correlated with these clinical variants, supporting the hypothesis that the mutilans form represents a final stage of aggressive polyarthritis. These findings align with the literature, which identifies these antigens as predictive markers of disease severity and progression.
For the distal interphalangeal, oligoarticular, and axial forms, although antigenic similarities exist, specific associations for each variant were identified. Antigens such as HLA-A2, HLA-A3, HLA-A5, HLA-A8, and HLA-B27, along with various combinations among them, were frequently observed, highlighting the differentiated role of genetic components in clinical manifestations.
The data in Table 1 illustrate the specificity of HLA antigens for each clinical variant of PsA, emphasizing the potential of HLA profiling in risk stratification and personalized treatment. Particularly for polyarticular and mutilans forms, early identification of these genetic associations can guide aggressive therapeutic interventions, preventing progression to severe disabilities.
Table 1. Associations of HLA antigens with clinical variants of PsA in patients with PsA associated with cutaneous psoriasis (group I) and without cutaneous psoriasis (group II) | |||
Clinical variant | General group | Group I | Group II |
Axial | HLA-B27 HLA-B27/A2 HLA-B27/A3 | HLA-B27 HLA-B27/A2 HLA-B27/A3 | HLA-B27/A2 HLA-B27/A3 |
Distal interphalangeal | HLA-A2/A3 HLA-A2/A5 HLA-A2/A8 HLA-B27/A3 HLA-A3/A8 | HLA-A2/A3 HLA-A2/A5 HLA-A2/A8 | HLA-B27/A3 HLA-A3/A8 |
Oligoarticular | HLA-B27/A3 HLA-A2/A5 HLA-A3/A8 HLA-A2/A3 | HLA-B27/A3 HLA-A2/A5 HLA-A3/A8 | HLA-B27/A3 HLA-A2/A3 |
Polyarticular | HLA-B27/B8 HLA-B27/B62 HLA-B27B11 | HLA-B27/B8 HLA-B27/B62 HLA-B27B11 | HLA-B27/B62 HLA-B27/B11 |
Mutilans | HLA-B27/B8 HLA-B27/B11 | HLA-B27/B8 | HLA-B27/B11 |
Note: HLA – human leukocyte antigen; PsA – psoriatic arthritis | |||
The study revealed significant correlations between HLA antigens and disease severity, as assessed by the DAPSA score (Table 2). In patients with PsA associated with cutaneous psoriasis, HLA-B27/B8, HLA-B27/B62, and HLA-B27/B11 antigens were linked to high DAPSA scores (≥50), indicating severe and progressive clinical forms. Conversely, HLA-A2/A5 and HLA-A3/B17 antigens were associated with moderate DAPSA scores (30-40), suggesting less aggressive forms. These findings confirm the impact of HLA antigens on disease activity and highlight their utility in risk stratification and guiding personalized treatment strategies.
Table 2. Factorial correlation of HLA antigens in patients with PsA associated with cutaneous psoriasis (group I) and without psoriasis (group II) with DAPSA scores | ||
| DAPSA ≥25 and <35 | DAPSA ≥35 |
Group I | HLA-B27/B32; HLA-A3/A8 | HLA-B27/B62; HLA-B27/B11; HLA-B27/B8; HLA-B62/B8 |
Group II | HLA-B27; HLA-A3/A8 | HLA-B27/B62; HLA-B27/B32; HLA-B27/B11 |
Note: HLA – human leukocyte antigen; PsA – psoriatic arthritis; DAPSA – disease activity index for psoriatic arthritis | ||
The research revealed significant correlations between HLA antigen variants and the clinical activity of PsA, as measured by the DAPSA score. The isolated HLA-B27 antigen was associated with DAPSA scores ranging from 25 to 35 (p<0.0001), reflecting moderate disease activity. However, when HLA-B27 was combined with antigens such as HLA-B32, HLA-B62, HLA-B11, and HLA-B8, it resulted in DAPSA scores of ≥50, indicating severe forms of the disease with pronounced articular inflammation. Conversely, combinations of HLA-B27 with HLA-A2 or HLA-A3 correlated with DAPSA scores below 20 (p<0.05), suggesting a protective effect.
In patients with PsA without cutaneous psoriasis, the results were similar. However, the influence of HLA-B27 was more variable across clinical forms, and the protective effects of combinations with HLA-A2 and HLA-A3 were less pronounced. These findings underscore the critical role of HLA antigens in determining disease severity and influencing evolutionary risk, providing a foundation for personalized patient evaluation.
The study also highlighted significant correlations between other HLA antigens and PsA activity, measured by the DAPSA score. Antigens from other categories were associated with DAPSA scores between 20 and 30 (p<0.0001), reflecting moderate disease activity. HLA-B27 stood out due to its association with high DAPSA scores, emphasizing its role in amplifying inflammation and disease severity. In contrast, HLA-A2 and HLA-A3 demonstrated a significant protective effect (p<0.01), contributing to reduced clinical activity.
HLA-A2 was identified as the most protective antigen (CI+95% = 302.8), followed by HLA-A5 (CI+95% = 243.6), HLA-A3 (CI+95% = 214.7), and HLA-A8 (CI+95% = 126.1). These findings highlight the importance of HLA antigen profiling in assessing risk and disease progression, offering a basis for personalized therapeutic interventions.
Discussions
PsA is a complex autoimmune disease influenced by genetic predisposition and environmental factors, which significantly contribute to its clinical variability [1, 5, 6]. Our research, consistent with previous studies, has demonstrated that HLA antigens play a pivotal role in disease severity and clinical phenotypes, emphasizing their potential as biomarkers for risk assessment and personalized treatment strategies [7-9]. Aggressive HLA antigens, such as HLA-B27, B8, B11, B32, and B62, were frequently associated with severe forms of the disease characterized by intense inflammation and extensive systemic involvement. In contrast, protective antigens like HLA-A2, A3, A5, and A8 correlated with reduced inflammatory activity and minimal joint damage.
Our analyses revealed significant differences between patients with cutaneous psoriasis and those without skin manifestations. Among patients with cutaneous psoriasis, HLA-B27/B62 and HLA-B27/A3 combinations were frequently identified and linked to mixed articular and cutaneous involvement. On the other hand, patients without cutaneous psoriasis exhibited distinct combinations, such as HLA-B27/B62 and HLA-B27/B11, suggesting a specific genetic profile for this subgroup. Factorial analysis highlighted HLA-B27/B8 and HLA-B27/B62 as key markers for severe PsA forms with DAPSA scores ≥50, while combinations of HLA-B27 with HLA-A2 and HLA-A3 demonstrated protective effects, being associated with lower DAPSA scores (<20).
The distribution of HLA antigens across clinical subtypes provides valuable insights into the disease's pathogenesis. For instance, HLA-B27 antigens predominated in the axial variant, underscoring their role in vertebral and sacroiliac joint inflammation. Other clinical variants, such as the polyarticular and mutilating forms, were marked by the presence of HLA-B27/B8 and HLA-B27/B11, indicating an increased risk for aggressive and destructive progression. HLA-A2 and HLA-A3 antigens were associated with less severe forms, suggesting potential protective mechanisms [6, 9-11].
These findings underscore the importance of HLA antigens as predictive tools and guides for personalized treatment in PsA. Integrating these results into clinical practice could significantly enhance diagnosis, monitoring, and disease management, reducing both long-term complications and the therapeutic burden on patients [2, 5, 12-14]. Furthermore, the application of modern technologies, such as artificial intelligence and machine learning algorithms, may enable more precise risk stratification and efficient therapeutic interventions, paving the way for improved patient outcomes in PsA management [7, 15, 16].
Conclusions
The study highlights the role of HLA antigens in the pathogenesis and severity of PsA, demonstrating significant correlations between aggressive HLA antigens (HLA-B27, B8, B11, B32, B62) and severe disease forms, as well as protective antigens (HLA-A2, A3, A5, A8) and less aggressive forms. Antigenic differences between patients with and without cutaneous psoriasis suggest distinct immunogenetic mechanisms, reinforcing the need for genetic profiling for precise risk stratification.
The association of HLA antigens with disease activity, measured by the DAPSA score, validates the use of these markers for personalized treatments and early interventions. These findings emphasize the importance of integrating advanced technologies into clinical practice to enhance patient prognosis and quality of life.
Competing interests
None declared.
Patient consent
Obtained.
Ethics approval
The study was approved by the Research Ethics Committee of Nicolae Testemițanu State University of Medicine and Pharmacy (No.21 from 21.12.2019).
Funding
The author has not declared a specific grant for the research from any funding agency in the public, commercial or not-for-profit sectors.
Provenance and peer review
Not commissioned, externally peer review.
Author’s ORCID ID
Eugeniu Russu – https://orcid.org/0000-0001-8957-8471
References
Griffiths CEM, Armstrong AW, Gudjonsson JE, Barker JNWN. Psoriasis. Lancet. 2021;397:1301-1315. doi: 10.1016/S0140-6736(20)32549-6.
Dand N, Mahil SK, Capon F, et al. Psoriasis and genetics. Acta Derm Venereol. 2020;100(3):adv00030. doi: 10.2340/00015555-3384.
Ogawa K, Okada Y. The current landscape of psoriasis genetics in 2020. J Dermatol Sci. 2020;99(1):2-8. doi: 10.1016/j.jdermsci.2020.05.008.
Lønnberg AS, Skov L, Skytthe A, et al. Heritability of psoriasis in a large twin sample. Br J Dermatol. 2013;169(2):412-416. doi: 10.1111/bjd.12375.
Singh S, Pradhan D, Puri P, et al. Genomic alterations driving psoriasis pathogenesis. Gene. 2019;683:61-71. doi: 10.1016/j.gene.2018.09.042.
Gudjónsson JE, Kárason A, Runarsdottir EH, et al. Distinct clinical differences between HLA-Cw*0602 positive and negative psoriasis patients – an analysis of 1019 HLA-C- and HLA-B-typed patients. J Invest Dermatol. 2006;126(4):740-745. doi: 10.1038/sj.jid.5700118.
Mak RK, Hundhausen C, Nestle FO. Progress in understanding the immunopathogenesis of psoriasis. Actas Dermo-sifiliogr. 2009;100(Suppl 2):2-13. doi: 10.1016/s0001-7310(09)73372-1.
Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361:496-509. doi: 10.1056/NEJMra0804595.
Gudjonsson JE, Karason A, Antonsdottir A, et al. Psoriasis patients who are homozygous for the HLA-Cw*0602 allele have a 2.5-fold increased risk of developing psoriasis compared with Cw6 heterozygotes. Br J Dermatol. 2003;148(2):233-235. doi: 10.1046/j.1365-2133.2003.05115.x.
Mallon E, Bunce M, Savoie H, et al. HLA-C and guttate psoriasis. Br J Dermatol. 2000;143(6):1177-1182. doi: 10.1046/j.1365-2133.2000.03885.x.
Szczerkowska-Dobosz A, Rebała K, Szczerkowska Z, Nedoszytko B. HLA-C locus alleles distribution in patients from northern Poland with psoriatic arthritis – preliminary report. Int J Immunogenet. 2005;32(6):389-391. doi: 10.1111/j.1744-313X.2005.00543.x.
Ho PY, Barton A, Worthington J, et al. HLA-Cw6 and HLA-DRB1*07 together are associated with less severe joint disease in psoriatic arthritis. Ann Rheum Dis. 2007;66(6):807-811. doi: 10.1136/ard.2006.064972.
Gudjónsson JE, Kárason A, Antonsdóttir AA, et al. HLA-Cw6-positive and HLA-Cw6-negative patients with Psoriasis vulgaris have distinct clinical features. J Invest Dermatol. 2002;118(2):362-365. doi: 10.1046/j.0022-202x.2001.01656.x.
Jin Y, Zhang F, Yang S, et al. Combined effects of HLA-Cw6, body mass index and waist-hip ratio on psoriasis vulgaris in Chinese Han population. J Dermatol Sci. 2008;52(2):123-129. doi: 10.1016/j.jdermsci.2008.04.016.
Eder L, Abji F, Rosen CF, et al. The association of HLA-class I genes and the extent of atherosclerotic plaques in patients with psoriatic disease. J Rheumatol. 2016;43(10):1844-1851. doi: 10.3899/jrheum.151469.
Li K, Huang CC, Randazzo B, et al. HLA-C*06:02 allele and response to IL-12/23 inhibition: results from the ustekinumab phase 3 psoriasis program. J Invest Dermatol. 2016;136(12):2364-2371. doi: 10.1016/j.jid.2016.06.631.