Introduction
Non-Hodgkin lymphoma (NHL) is a heterogeneous group of malignant lymphoid tumors [1, 2] and is one of the most common malignant lymphoproliferative disorders [3]. According to the results of the GLOBOCAN 2020 study, the incidence of new NHL cases is expected to increase from approximately 544,000 in 2020 to 778,000 by 2040, representing an estimated increase of 43% over two decades [4]. The 5-year prevalence rate of NHL in the Republic of Moldova is approximately 21 cases per 100,000 population [5]. Patients with NHL are prone to develop venous thromboembolism (VTE), which is the second leading cause of mortality among them [6]. In particular, the risk of VTE is further increased in patients undergoing chemotherapy [7]. VTE is induced by the complex interaction of various factors with endogenous or exogenous procoagulant action. [8-10]. It is indisputable that the risk of VTE increases according to the totality of risk factors assessed directly in each individual patient on basis on age, gender, comorbidities, Eastern Cooperative Oncology Group (ECOG), and congenital and acquired thrombophilia [10]. Hemostasis disorders associated with NHL develop severe complications, limit treatment options and their results, and alter quality of life [11, 12]. It is difficult, but absolutely necessary, to predict the risk of thrombosis in asymptomatic carriers of anticardiolipin (aCL), anti-β2-glycoprotein I (anti-β2GP I), and lupus anticoagulant (LA) antibodies, and risk stratification is a fundamental element of current medical research, including in patients with NHL [13]. Seropositivity of these antibodies in malignancies could remain asymptomatic.
As personalized treatment of NHL based on new molecules continues to improve survival rates, there is an urgent need to address the associated risks of thrombosis and bleeding. Assessing the risk of developing hemostatic disorders and subsequent stratification with individual customization for each patient with NHL is absolutely necessary.
The aim of this study was to evaluate the incidence of hemostatic disorders according to age, gender, NHL type, degree of dissemination, B symptoms, disease onset, tumor size, positivity of aCL, anti-β2GP I and LA antibodies, fibrinogen level, LDH and ECOG performance status.
Material and methods
Within the Oncology Institute of the Republic of Moldova conducted the prospective, descriptive study (2020-2024) with the inclusion of 161 de novo patients with aggressive (56.5%) and indolent (43.5%) NHL. The research protocol, information, and acceptance forms were approved by the Research Ethics Committee of Nicolae Testemițanu State University of Medicine and Pharmacy (no. 32 of 28.01.2020). The scientific research was conducted with the support of the National Agency for Research and Development, within the Postdoctoral Programs, project number 24.00208.8007.02/PD.
The inclusion criteria were: age over 18 years, an immunohistochemically confirmed diagnosis of NHL, the patient's consent to participate in the study, and the possibility of dynamic monitoring. The respondents were comprehensively evaluated using clinical, paraclinical, and imaging methods to assess the NHL type, stage, onset of the disease (nodal/extranodal), tumor size, and B symptoms. aCL IgM and IgG, anti-β2GP I IgM and IgG antibodies were measured by enzyme-linked immunosorbent assay (ELISA), and LA by the turbidimetry method. Quantitative testing of D-dimers was performed by automatic latex agglutination with photometric detection, with reference values of <0.5 μg/mL. Plasma fibrinogen levels were assessed by coagulometry, with reference values of 200-400 mg/dL (2.0-4.0 g/L). LDH was assessed by spectrophotometric method.
The development and location of thromboses were confirmed by radiological evidence, including venous ultrasonography, computed tomography, or conventional angiography, depending on the anatomical location.
To achieve the proposed goal, the database of the accumulated material was statistically processed using Microsoft Excel, GraphPad Prism ver. 9.3.0, Epi Info 7.2, EpiMax Table, and IBM SPSS Statistics version 26.0. The Mann-Whitney U test was used to compare 2 groups without assuming that the studied values were normally distributed, using the null hypothesis that the medians of two samples were identical. Multiple regression and logistic regression were applied by calculating the odds ratio (OR) and the 95% confidence interval (CI).
Results
According to the eligibility criteria, 161 patients with NHL were included in the study: 84 (52%) men (95% CI, 44-60) and 77 (48%) women (95% CI, 40-56), aged between 24 and 82 years, with a median age of 59 years. In our study, patients with aggressive NHL (91; 56.5%; 95% CI, 48-64) predominated over those with indolent NHL (70; 43.5%; 95% CI, 36-52), with a higher prevalence of advanced stages (III and IV) in 106 cases (65.8%; 95% CI, 58-73) (p < 0.001) and B symptoms in 50.3% of cases (95% CI, 42–58) (p = 0.5). Extranodal onset in aggressive NHL had approximately the same frequency as in indolent NHL: 33 cases (20.5%; 95% CI, 15-28) versus 37 cases (23%; 95% CI, 17-30) (p = 0.035).
Table 1. Characteristics of the research group | |
Parameter | Patients (n, %, 95% CI) |
Age range (years) | 24-82 |
Gender Women Men |
77 (48%) (95% CI, 40-56) 84 (52%) (95% CI, 44-60) |
Types of NHL Aggressive Indolent |
91 (56.5%) (95% CI, 48-64) 70 (43.5%) (95% CI, 36-52) |
Cell substrate B T |
157 (97.5%) (95% CI, 93-99) 4 (2.5%) (95% CI, 0.80-6.6) |
NHL stage Localized (I-II) Advanced (III-IV) |
55 (34.2%) (95% CI, 27-42) 106 (65.8%) (95% CI, 58-73) |
Symptoms A B |
80 (49.7%), (95% CI, 42-58) 81 (50.3%), (95% CI, 42-58) |
Onset of the disease Nodal Extranodal |
91 (56.5%) (95% CI, 49-64) 70 (43.5%) (95% CI, 36-52) |
Note: NHL - non-Hodgkin lymphoma, CI - confidence interval. | |
Hemostatic disorders were identified in 17 patients (10.6%) (95% CI, 6.3%-16%), with thrombotic events occurring in 11 (6.7%) (95% CI, 3.5%-12%) (p = 0.12), compared to 6 (3.9%) (95% CI, 1.4%-8%) hemorrhagic events, in a ratio of 1.8:1. All thrombotic events observed exclusively in the venous system, affecting patients with aggressive NHL in 9 cases (4.3%), (95% CI, 3%-10%) versus patients with indolent NHL in 2 cases (1.6%) (95% CI, 0.2%-4.4%), with a ratio of 4.5:1. The relative risk (RR) was 1.5, and the Odds Ratio (OR) was 3.7; however, the difference did not reach statistical significance in this study (Fisher's exact test, p = 0.11).
Venous thromboembolism occurred in the deep veins of the lower extremities in 4 cases (2.4%) (95% CI, 0.7%-6.2%), in the jugular vein in 4 cases (2.4%) (95% CI, 0.7%-6.2%), in the deep veins of the upper extremities in 1 case (0.6%) (95% CI, 0.02%-3.4%), in the portal vein in 1 case (0.6%) (95% CI, 0.02%-3.4%), and in subclavian vein in 1 case (0.6%) (95% CI, 0.02%-3.4%).
VTE was more frequent in men–9 cases (82%) (95% CI, 48%-97%)–compared to women–2 cases (18%) (95% CI, 8%-19%) (p = 0.041). This finding suggests that the male gender could be considered a risk factor for thrombosis.
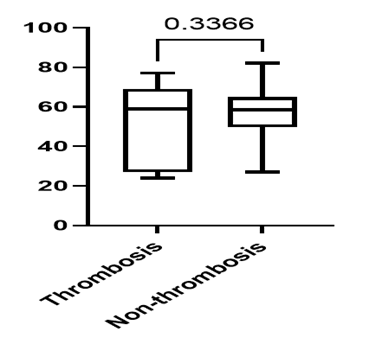
The age of NHL patients who developed thrombotic complications ranged from 24 to 77 years, with a mean age of 50 years, whereas the age of non-thrombotic NHL patients ranged from 27 to 79 years, with a mean age of 57 years (p = 0.3366) (Fig. 1).
|
Fig. 1 Distribution of NHL patients according to age and the presence or absence of thrombosis . |
The average interval between thrombosis and the diagnosis of NHL was 3-4 weeks in 7 (4.3%) (95% CI, 1.8%-8.8%) cases. In 4 (2.4%) (95% CI, 0.7%-6.2%) NHL patients, a thrombotic event developed during first-line treatment, with an average onset of 9 months.
Positivity for aCL, anti-β2GPI, and LA antibodies was recorded in 26 patients with B-cell NHL, accounting for 16.2% (95% CI, 10.8%-23%) of cases. Single positivity was observed in 23 (14.3%) (95% CI, 9.3%-21%) cases, double positivity in 2 (1.3%) (95% CI, 0.2%-4.4%) cases, and triple positivity in 1 (0.6%) (95% CI, 0.02%-3.4%) case. Double positivity was represented by the combinations aCL IgM + LA and aCL IgM + anti-β2GPI IgM. Triple positivity was characterized by the association of aCL IgM + LA + anti-β2GPI IgG.
Only 6 (3.7%) (95% CI, 1.4%-8%) of the patients who developed thrombotic complications tested positive for aCL, anti-β2GPI, and LA antibodies. Among the 11 (6.7%) (95% CI, 3.5%-12%) NHL patients with thrombosis, 3 (1.8%) (95% CI, 0.4%-5.4%) had single antibody positivity (2 with LA and 1 with anti-β2GPI IgM); 2 (1.3%) (95% CI, 0.2%-4.4%) had double antibody positivity (aCL IgM + LA and aCL IgM + anti-β2GPI IgM); and 1 (0.6%), (95% CI, 0.02%-3.4%) had triple antibody positivity (LA + aCL IgM + anti-β2GPI IgG).
The primary involvement of mediastinal nodes in patients who developed thrombosis was 45.5%, compared to non-mediastinal involvement in 54.6%. In contrast, among patients with NHL without thrombosis, mediastinal node involvement was observed in 12.5%, while non-mediastinal involvement was seen in 76%. These results were statistically significant with a p-value of 0.02, as assessed by the Fisher exact test. The relative risk (RR) of thrombosis association is 1.3 (95% CI, 1.04%-1.98%), and the Odds Ratio (OR) is 5.069 (95% CI, 0.34%-16.8%).
Nodal tumor sizes ≥ 7 cm were predominant in 8 (4.9%) (95% CI, 2.2%-10%) cases out of 11 (6.7%) (95% CI, 3.5%-12%) NHL patients with associated VTE, although the difference did not reach statistical significance (Fisher exact test, p = 0.1). Among these, 6 (3.7%) (95% CI, 1.4%-8%) patients had Diffuse large B-cell lymphoma, and 1 (0.6%) (95% CI, 0.02%-3.4%) patient had NHL of gray zone cells, and 1 (0.6%) (95% CI, 0.02%-3.4%) patient had small lymphocytic lymphoma.
No statistical difference was found between cases with and without thrombosis regarding LDH levels (p = 0.69, Mann-Whitney U test).
A high level of fibrinogen was found in 39 (24.4%) (95% CI, 18%-32%) patients: 30 (18.8%) (95% CI, 13%-26%) cases in aggressive NHL and 9 (5.6%) (95% CI, 3%-10%) cases in indolent NHL, with a ratio of 3.3:1.Notably, patients with a confirmed thrombotic event had a higher level of fibrinogen at diagnosis (median, 4.2; mean, 4.06; 95% CI of mean, 3.4%-4.7%) compared to those who did not manifest thrombotic events (median, 3.1; mean, 3.4; 95% CI of mean, 3.1%-3.5%), suggesting a potential association between fibrinogen levels at diagnosis and the risk of thrombosis in lymphoma patients (Mann-Whitney U test, p = 0.02) (Fig. 2).
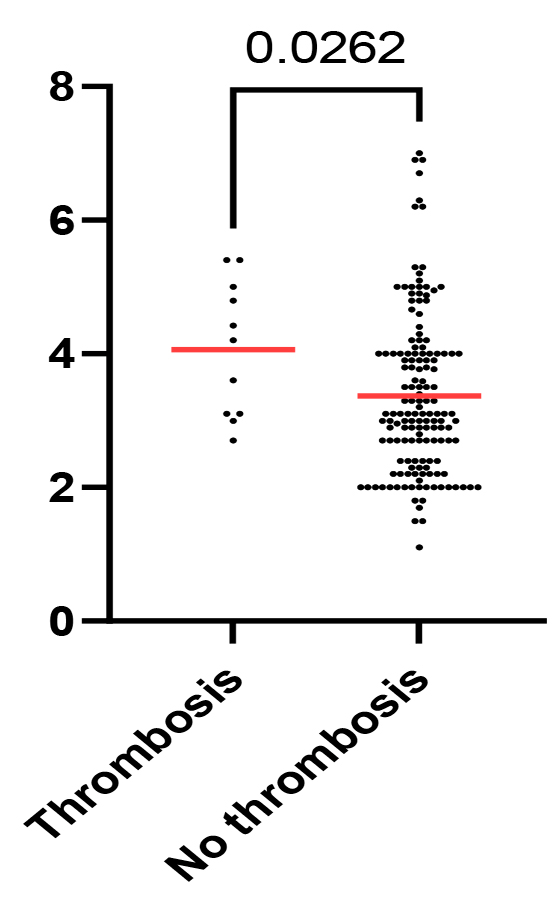 |
Fig. 2 Scatter plot of fibrinogen levels across patients with and without thrombotic events. P-value shown for the Mann-Whitney U test. |
The analysis of the distribution of patients according to the level of D-dimers and subtype of NHL reveals high values (≥ 501 ng/ml) in 45 patients (28%) (95% CI, 21%-36%), including those with aggressive NHL – 31 cases (19.3%) (95% CI, 13%-26%) and indolent NHL – 14 cases (8.7%) (95% CI, 3%-10%) (p = 0.0015, Mann-Whitney U test).
The distribution of patients with NHL and VTE according to ECOG performance status shows an ECOG score of 2-4 in 7 patients (4.2%) (95% CI, 1.8%-8.8%) and an ECOG score of 0-1 in 4 patients (2.5%) (95% CI, 0.7%-6.2%). Statistical significance was not reached in the thrombosis analysis according to ECOG (p = 0.1, Fisher's exact test).
Discussion
The current maximally personalized treatment of NHL allows for life prolongation and even cure; however, complications associated with the disease and treatment methodology inevitably have a major impact on quality of life. Among these complications are hemostasis disorders, which limit treatment options and negatively affect patients’ quality of life [11, 12].
A meta-analysis conducted by Caruso and colleagues indicated that the overall incidence rate of thrombosis in non-Hodgkin lymphoma patients is 6.4% [14]. A similar result was obtained in our study, with thrombotic events found in 6.7% of cases. According to the research results by Hohaus and colleagues, aggressive NHL is more often associated with thrombotic complications than indolent NHL, with VTE occurring in 10-15% of cases within the first year of diagnosis [15]. Similar conclusions were drawn from our research, which found thrombosis in 4.3% of aggressive NHL cases compared to 1.6% of indolent NHL cases.
An American study analyzed data from 16,755 patients with aggressive and indolent NHL and found that age over 45 years at baseline is already a risk factor for VTE in NHL patients [16]. The average age of patients in our study who developed NHL-associated thrombosis was 50 years, which does not significantly differ from the findings of other studies.
Female gender as a potential risk factor for severe grade III VTE was assessed in a multicenter study conducted in Italian hematology clinics [17]. In our study, we obtained different results: VTE developed more often in men (82%) than in women (18%) (p = 0.041), suggesting that male gender may be considered a risk factor for thrombosis.
Retrospective studies analyzed and described by Razak demonstrate differences in both gender and thrombosis type (arterial vs venous). According to the authors, women are at higher risk of venous thromboembolism, while men are more susceptible to arterial thromboembolism [18]. In our study, thrombotic events were assessed only in the venous system.
The location and size of the tumor in NHL increase the risk of thrombotic events through external compression of large blood vessels. Serbian researchers found that mediastinal and extranodal lymphoma onset are major risk factors for thromboembolism [19]. According to Yuen’s findings, mediastinal involvement was associated with an eightfold higher risk of VTE, while extranodal locations, such as the central nervous system, testis, and gastrointestinal tract, increased the risk of VTE by 2.3-fold [20]. In our study, primary mediastinal nodes involvement was observed in 45.5% of patients who developed thrombosis, compared to 54.6% cases with non-mediastinal involvement. Among NHL patients without thrombosis, mediastinal involvement was observed in 12.5% compared to 76% with non-mediastinal involvement. These results were statistically significant (p = 0.02, Fisher’s exact test).
The risk of VTE is elevated during the first two months after lymphoma diagnosis and decreases over time [15]. Similar results were reported in a prospective Spanish study, where VTE developed within the first 90 days after diagnosis and initiation of antitumor therapy in 9.5% of patients with malignant lymphomas and multiple myeloma [21]. In our study, thrombotic complications occurred between 3-4 weeks in 4.3% of cases to 9 months in 2.4% of cases.
According to Barreno-Rocha, the synthesis of phospholipid antibodies by tumor cells serves as a target for aCL, LA, and anti-β2GPI [22]. In the global population, the prevalence of aPL antibodies varies between 1-5% and increases with chronic inflammatory and infectious diseases, as well as with the development of oncological conditions [23, 24]. Our study demonstrates a 16.2% incidence of aCL, LA, and anti-β2GPI antibodies in newly diagnosed NHL patients. A lower prevalence of autoantibodies–9 (41%) in NHL patients–was reported by Sciarra et al. in 1995 [25]. In our cohort, LA was the most prevalent, being positive in 21 (13.1%) cases, followed by anti-β2GPI IgM and aCL IgM antibodies, each found in 4 (2.5%) cases. A higher prevalence (40%) of anti-β2GPI IgM was reported among 86 NHL patients treated at the Institute of Hematology in Israel [26].
A major risk for thrombosis is suspected not only when an individual antibody is detected but also when an association of 2-3 antibody types is present, regardless of their IgG or IgM isotype. This is referred to as the "aPL double profile" and "aPL triple profile", respectively [27].
Niimi and colleagues aimed to assess the clinical utility of increasing the D-dimer cut-off value by evaluating this parameter in 208 patients with malignancies, including those with NHL. The study results highlighted an optimal cut-off value of 4.0 μg/mL for the diagnosis of DVT in patients with malignancy. Additionally, the study suggested that combining the Khorana score with D-dimer levels provided a more accurate diagnosis of DVT than the Khorana score alone [28].
A decrease in ECOG performance status (2-4) was associated with a more proximal localization of VTE, particularly in the lower limbs [29]. This relationship between low ECOG performance status and thrombosis was confirmed in a study conducted by Hohaus. In our study, the distribution of NHL patients with VTE according to ECOG performance status showed a higher prevalence of ECOG 2-4 in 7 (4.2%) cases compared to ECOG 0-1 in 4 (2.5%) cases.
Focusing on these antibodies could lead to better management of NHL patients by aiding in prediction, ultimately improving overall survival and quality of life.
Conclusions
These differences suggest that not all seropositive NHL subjects develop thrombosis, and it is possible that some NHL patients with thrombosis are seronegative for aCL, anti-β2GPI, and LA antibodies. Patients with tumor conglomerates ≥ 7 cm, regardless of aCL, anti-β2GPI, and LA antibody positivity, but with mediastinal localization, present the highest risk of developing thrombotic complications.
The prevalence of aCL, anti-β2GPI, and LA antibodies in NHL patients was appreciated in 16.2% of cases. This is expressed as single-positivity in 14.3%, double positivity in 1.3%, and triple positivity in 0.6%, exclusively in B-cell NHL. A statistically significant difference in antibody positivity was observed based on age and NHL type. However, antibody synthesis in NHL patients showed no statistically significant association with gender, disease dissemination, B symptoms, or the location of the primary tumor focus. The risk of VTE in NHL is influenced by gender, NHL type, tumor size, mediastinal onset, hyperfibrinogenemia, antibody synthesis. However, no statistically significant association was found between VTE occurrence in NHL patients and disease stage, B symptoms, LDH levels or ECOG performance status.
Competing interests
None declared.
Authors’ contributions
SB and MM played a crucial role in the collection and analysis of empirical data, laying the foundation for the central argument of the paper. Their meticulous work enabled not only a novel interpretation of the data but also its integration into the broader context of specialist research. All authors have read and approved the final version of the manuscript.
Ethics approval
The study was approved by the Research Ethics Committee of Nicolae Testemițanu State University of Medicine and Pharmacy (Minutes No. 32, dated 28.01.2020).
Patient consent
Obtained.
Acknowledgements and funding
The study was supported by the National Agency for Research and Development of the Republic of Moldova within the Postdoctoral Programs, project number 24.00208.8007.02/PD.
Provenance and peer review
Not commissioned, externally peer reviewed.
Authors’ ORCID IDs
Sanda Buruiană – https://orcid.org/0000-0003-2341-0099
Minodora Mazur – https://orcid.org/0000-0003-4562-1452
References
Singh R, Shaik S, Negi BS, et al. Non-Hodgkin's lymphoma: a review. J Family Med Prim Care. 2020;9(4):1834-40. doi: 10.4103/jfmpc.jfmpc_1037_19.
Tomacinschii V, Buruiană S, Robu M. Clinical application of HALP score in the determination of nodal non-Hodgkin lymphoma prognosis. Doc Haematol - Rev Rom Hematol. 2023;1(2):51-58. https://doi.org/10.59854/dhrrh.2023.1.2.51.
Lazar S, Popovici D, Sarau O, et al. The experience of Timisoara Hematology Clinic on the management of aggressive non-Hodgkin lymphoma patients. Doc Haematol - Rev Rom Hematol. 2024;2(2):65-73. https://doi.org/10.59854/dhrrh.2024.2.2.65.
Chu Y, Liu Y, Fang X, et al. The epidemiological patterns of non-Hodgkin lymphoma: global estimates of disease burden, risk factors, and temporal trends. Front Oncol. 2023;13:1059914. https://doi.org/10.3389/fonc.2023.1059914.
World Health Organization, International Agency for Research on Cancer (IARC). Global Cancer Observatory. Republic of Moldova fact sheets. Lyon: IARC; 2021.
Farge D, Frere C, Connors JM, Ay C, et al. 2019 International clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. Lancet Oncol. 2019;20(10):e566-81. https://doi.org/10.1016/S1470-2045(19)30336-5.
Islam MA. Antiphospholipid antibodies and antiphospholipid syndrome in cancer: uninvited guests in troubled times. Semin Cancer Biol. 2020;64:108-113. https://doi.org/10.1016/j.semcancer.2019.07.019.
Solinas C, Saba L, Sganzerla P, Petrelli F. Venous and arterial thromboembolic events with immune checkpoint inhibitors: a systematic review. Thromb Res. 2020;196:444-453. https://doi.org/10.1016/j.thromres.2020.09.038.
Mukai M, Oka T. Mechanism and management of cancer-associated thrombosis. J Cardiol. 2018;72(2):89-93. https://doi.org/10.1016/j.jjcc.2018.02.011.
Buruiană S. Managementul riscului complicațiilor tromboembolice în oncologie [Risk factors of thromboembolic complications in oncology]. Public Health Econ Manag (Chișinău). 2021;(1):57-62. https://doi.org/10.52556/2587-3873.2021.1(88).07. Romanian.
Bønløkke S, Fenger-Eriksen C, Ommen H, Hvas A. Impaired fibrinolysis and increased clot strength are potential risk factors for thrombosis in lymphoma. Blood Adv. 2023;7(22):7056-7066. doi: 10.1182/bloodadvances.2023011379.
Khorana AA, Mackman N, Falanga A, et al. Cancer-associated venous thromboembolism. Nat Rev Dis Primers. 2022;8(1):11. doi: 10.1038/s41572-022-00336-y.
Aguirre del-Pino R, Monahan R, Huizinga T, et al. Risk factors for antiphospholipid antibodies and antiphospholipid syndrome. Semin Thromb Hemost. 2024;50(6):817-828. doi: 10.1055/s-0043-1776910.
Caruso V, di Castelnuovo A, Meschengieser S, Lazzari MA, et al. Thrombotic complications in adult patients with lymphoma: a meta-analysis of 29 independent cohorts including 18 018 patients and 1149 events. Blood. 2010;115(26):5322-5328. doi: 10.1182/blood-2010-01-258624.
Hohaus S, Bartolomei F, Cuccaro A, et al. Venous thromboembolism in lymphoma: risk stratification and antithrombotic prophylaxis. Cancers. 2020;12(5):1-17. doi: 10.3390/cancers12051291.
Mahajan A, Brunson A, Adesina O, Keegan THM, Wun T. The incidence of cancer-associated thrombosis is increasing over time. Blood Adv. 2022 Jan 11;6(1):307-320. doi: 10.1182/bloodadvances.2021005590.
Santi RM, Ceccarelli M, Bernocco E, et al. Khorana score and histotype predicts incidence of early venous thromboembolism in non-Hodgkin lymphomas: a pooled-data analysis of 12 clinical trials of fondazione italiana linfomi (FIL). Thromb Haemost. 2017;117(8):1615-1621. doi: 10.1160/TH16-11-0895.
Razak NB, Jones G, Bhandari M, Berndt MC, Metharom P. Cancer-associated thrombosis: an overview of mechanisms, risk factors, and treatment. Cancers. 2018;10(10):380. doi: 10.3390/cancers10100380.
Antic D, Ajtić O, Djikić D, et al. P1653: Inflammation mediated thrombus formation in lymphomas. HemaSphere. 2023;7(Suppl):e541657a.doi: 10.1097/01.HS9.0000973484.54165.7a.
Yuen HL, Slocombe A, Heron V, et al. Venous thromboembolism in primary central nervous system lymphoma during frontline chemoimmunotherapy. Res Pract Thromb Haemost. 2020;4(6):997-1003. doi: 10.1002/rth2.12415.
Sánchez Prieto S, Gutiérrez Jomarrón I, Martínez Vázquez C, et al. Comprehensive evaluation of genetic and acquired thrombophilia markers for an individualized prediction of clinical thrombosis in patients with lymphoma and multiple myeloma. J Thromb Thrombolysis. 2024;57(6):984-995. https://doi.org/10.1007/s11239-024-02977-0.
Barreno-Rocha SG, Guzmán-Silahua S, Rodríguez-Dávila SD, et al. Antiphospholipid antibodies and lipids in hematological malignancies. Int J Mol Sci. 2022;23(8):4151. https://doi.org/10.3390/ijms23084151.
Kungwankiattichai S, Nakkinkun Y, Owattanapanich W, Ruchutrakool T. High incidence of antiphospholipid antibodies in newly diagnosed patients with lymphoma and a proposed aPL predictive score. Clin Appl Thromb Hemost. 2020;26:1076029620928392. https://doi.org/10.1177/1076029620928392.
Buruiana S. Incidența anticorpilor antifosfolipidici la pacienții primary cu Limfom non-Hodgkin [Incidence of antiphospholipid antibodies in new patients with non-Hodgkin lymphoma]. Public Health Econ Manag (Chișinău). 2021;(4):34-38. https://doi.org/10.52556/2587-3873.2021.4(91).34-38. Romanian.
Sciascia S, Montaruli B, Infantino M. Antiphospholipid antibody testing. Med Clín (Barc). 2024;163(Suppl 1):S4-S9. https://doi.org/10.1016/j.medcli.2024.06.002.
Bairey O, Blickstein D, Monselise Y, et al. Antiphospholipid antibodies may be a new prognostic parameter in aggressive non-Hodgkin's lymphoma. Eur J Haematol. 2006;76(5):384-91. https://doi.org/10.1111/j.1600-0609.2005.00620.x.
Chayoua W, Kelchtermans H, Gris JC, et al. The (non‐)sense of detecting anti‐cardiolipin and anti‐β2glycoprotein I IgM antibodies in the antiphospholipid syndrome. J Thromb Haemost. 2020;18(1):169-179. https://doi.org/10.1111/jth.14633.
Niimi K, Nishida K, Lee C, Ikeda S, Kawai Y, Sugimoto M, Banno H. Optimal D-dimer cutoff values for diagnosing deep vein thrombosis in patients with comorbid malignancies. Ann Vasc Surg. 2024;98:293-300. doi: 10.1016/j.avsg.2023.06.033.
Hohaus S, Tisi M, Bartolomei F, et al. Risk factors for venous thrombembolism in patients with lymphoma requiring hospitalization. Blood Cancer J. 2018;8(6):54. doi: 10.1038/s41408-018-0096-1.