Introduction
Colorectal cancer (CRC) is one of the most common malignancies, being the second most common type of cancer in women and the third most common in men. Although many advances have been made in oncological and surgical treatment, and through the implementation of screening programs, CRC still remains first among malignant causes of death [1]. This condition is slowly progressive, developing over time. Most cases of CRC begin at the level of polyps in the epithelium of the colon and rectum, but there are other predisposing causes, such as inflammatory bowel diseases and certain hereditary genetic changes [2]. Approximately 80% of CRC cases have colorectal adenomatous polyps as a precursor [3], demonstrating the enormous benefit brought by screening patients for the detection and early removal of primary colonic neoformations (PCN). The terms” primary neoformations” and ”incipient neoplasia” of the colon were formulated in 1983 by the Japanese Society for the Study of Colorectal Cancer as tumors of the colon that are limited to the mucosa and submucosa, without the presence of secondary lesions, local, or distant metastases (Japanese Research Society for Cancer of the Colon and Rectum, 1983) [4]. CRC occurs through the malignant and uncontrolled transformation of the cells of the lining mucosal epithelium of the rectum and colon. Most colorectal carcinomas develop from preexisting adenomas. This concept of carcinogenesis is called the adenoma–carcinoma sequence. Progression from adenoma to colorectal carcinoma occurs through successive genetic abnormalities and mutations, involving oncogenes and tumor suppressor genes [5, 6]. The evolution from normal colonic epithelium to adenoma and carcinoma extends over several years, providing a sufficiently long period during which, through the use of diagnostic methods, precancerous lesions can be detected [7, 8].
The aim of this article is to evaluate the histopathological aspects and variants of primary colon neoplasms in correlation with their location and morphological characteristics.
Material and methods
The research presents a prospective clinical study based on the analysis of the treatment results of 255 patients with colonic and rectal neoformations, treated in the Nicolae Anestiadi Surgery Department No.1, Institute of Emergency Medicine, during the period 2018–2022. The study was approved by the Research Ethics Committee of the Nicolae Testemițanu State University of Medicine and Pharmacy, No. 52 dated 16.03.2018 and No. 7 dated 18.05.2022. The representative sample was calculated in the EpiInfo Program 7.2.2.6, "Stat Calc-Sample Size and Power" section, based on the following parameters: a confidence interval for 95% significance of the results, statistical power of 80%, the presence of colonic neoplasms in the population being 27% [Bray F. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries], and effect design = 4 (symptoms, laboratory paraclinical data, endoscopic examinations, and histological type of the formation). The calculated value is 216, with adjustment for the non-response rate, estimated at 10%.
The mean age was 61.3 ± 1.05 years. There were 145 (56.9%) men and 110 (43.1%) women, with a male to female ratio of 1.31:1. To conduct the study, the demographic data of the patients, operative protocols, endoscopic investigation protocols, histopathological reports, and multidisciplinary clinical, paraclinical, operative, and histopathological evaluations were collected and processed within the departments of Surgery, Endoscopy, and Pathological Anatomy. Regarding the morphopathological characteristics of colonic neoformations, the following qualitative nominal variables were analyzed: location, number, size, tumor appearance, histopathological type, and degree of tumor extension.
For the research, three groups were created based on the morphological results and the method of case management, with 58 respondents per group, in compliance with the study’s inclusion and exclusion criteria:
- Group I – patients with colorectal polyps
- Group II – patients with colorectal cancer in early stages (stages I-II)
- Group III – patients with colorectal cancer in advanced stages (stages III-IV).
To ensure greater accuracy, a series of inclusion and exclusion criteria were applied, thereby refining the study and focusing on a specific representative group.
Inclusion criteria for the research group:
Patients with colonic polypoid formations examined colonoscopically
Individuals over 18 years of age
Patients who provided informed consent to participate in the study
Patients whose mental state allows participation in the study.
Exclusion criteria were:
Patients hospitalized in emergency settings without endoscopic examination
Patients with malignancies in other locations or a history of surgery for any type of cancer
Patients with previously treated colorectal cancer (surgery, chemotherapy, or radiotherapy)
Patients with immunological disorders
Patients who declined participation in the study.
Statistical results were generated and processed using the R Studio program. The following descriptive statistics were estimated for numerical variables: minimum value, maximum value, mean value with standard deviation, and median value with interquartile range. For all statistical tests applied in the study, the significance threshold (p) was set at 0.05, complemented by 95% confidence intervals for relative frequencies.
Results
Through imaging, colonoscopy, and intraoperative methods, 255 patients with colonic neoformations were identified. Among these, 77 (30.2%) patients were diagnosed with malignant neoplasms of the colon or rectum, exhibiting various histological types, while 178 (69.8%) patients were diagnosed with precursor lesions of malignancy. The distribution of colonic neoformations by location is presented in Table 1.
Table 1. Distribution of colorectal neoplasms and premalignant lesions by location | ||||
Location | Right colon (abs, %) | Left colon (abs, %) | Rectum (abs, %) | Total (abs, %) |
Premalignant lesions | 52 (20.4%) | 78 (30.6%) | 48 (18.8%) | 178 (69.8%) |
Neoplasm | 35 (13.7%) | 26 (10.2%) | 16 (6.3%) | 77 (30.2%) |
Total | 87 (24.1%) | 104 (40.8%) | 64 (25.1%) | 255 (100%) |
Note: abs - absolute value in percentage | ||||
It was found that there are no significant differences in lesion distribution between patients with colorectal neoplasms and those with premalignant lesions (p > 0.05). The left colon has the highest proportion of premalignant lesions (30.6%), followed by the right colon (20.4%) and the rectum (18.8%). Overall, premalignant lesions are relatively evenly distributed among the three locations but predominate in the left colon, suggesting that this site may be more prone to such lesions. Neoplasms are more common in the right colon (13.7%) but have a lower incidence in the left colon (10.2%) and rectum (6.3%).
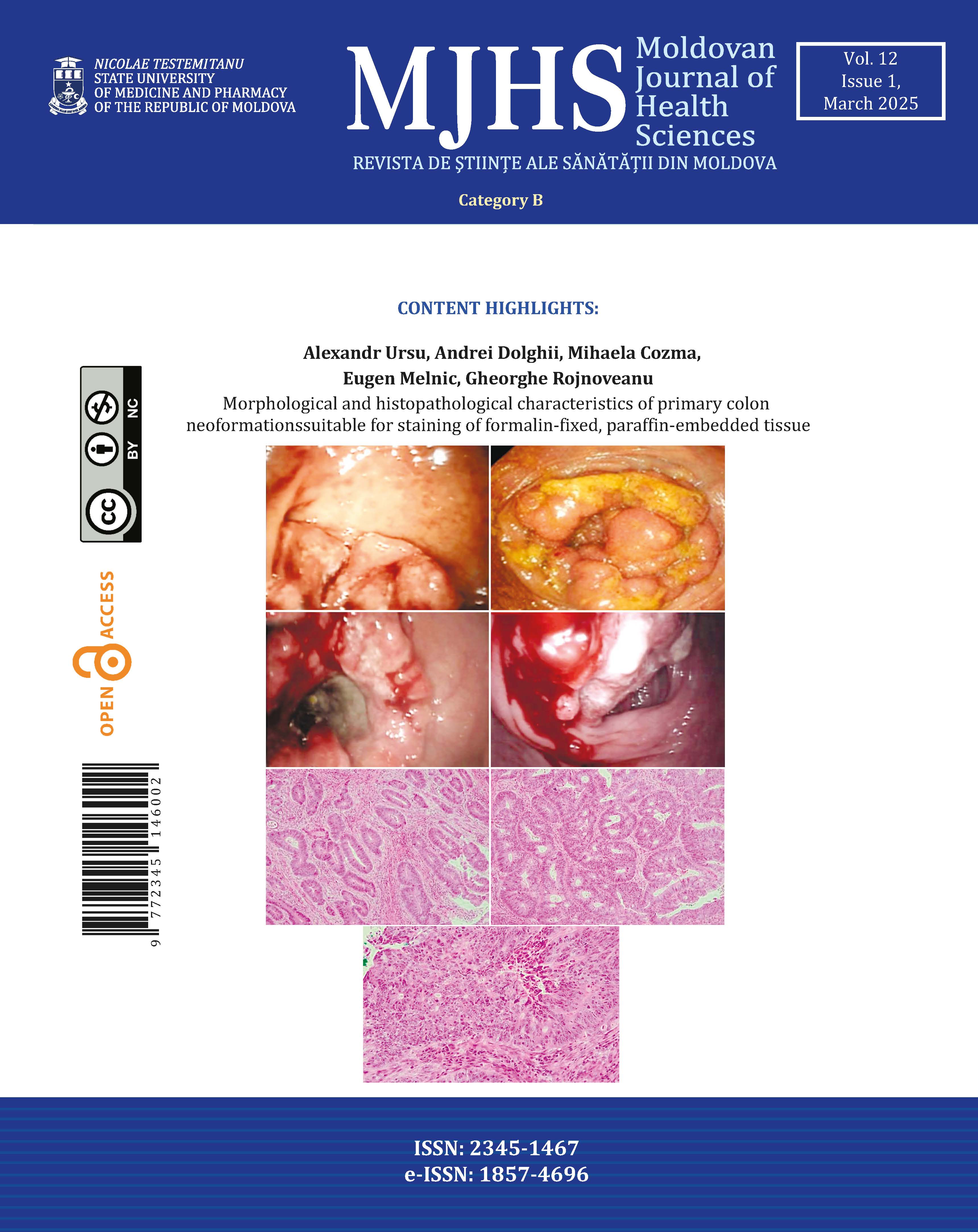
Regarding the endoscopic aspect, colorectal neoplasm presented in the following forms: vegetative formations accounted for 54.6% (n=42) of the 77 patients, vegetative-ulcerative tumor formations were observed in 24.6% (n=19), infiltrative formations with areas of ulceration were described endoscopically in 10.4% (n=8), while the classic infiltrative tumor aspect was detected in 8 patients (10.4%), as summarized in Figure 1.
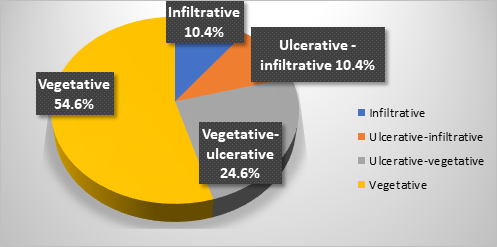 |
Fig. 1 Endoscopic appearance of colonic neoplasia in patients included in the study |
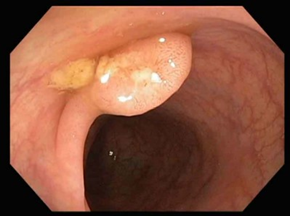
Figure 2 shows endoscopic images that reflect the appearance of different forms of colorectal tumors, all of which require histopathological confirmation. The ulcerated type, the most common, appears as a circular mass with a raised, irregular, and externalized border. It is frequently large, occupying an extensive portion of the colonic circumference. The polypoid form presents as a large mass protruding into the lumen. In 10% of cases, it has a mucinous structure characteristic of colloid carcinoma. The annular or stenotic form occupies the entire colonic lumen, with variable extension along the longitudinal axis of the colon.
|
Fig. 2. Endoscopic images of colorectal tumors A – vegetative formation; B – circumferential stenosing vegetative formation, impassable with the colonoscope; C – ulcer-infiltrative formation; D – vegetative formation covered by areas of necrosis |
In the present research, microscopic histological examination revealed that various variants of adenocarcinoma clearly dominate (100% of all identified tumors). Among them, conventional adenocarcinoma has the highest incidence, accounting for 87.1%. Table 2 presents the histological typology of colonic neoplasia.
Table 2. Histological typology of colonic neoplasia in CRC patients included in the study | |
Histological typology of the tumor | Cases (abs, %) |
Conventional adenocarcinoma | 67 (87.1%) |
Mucinous adenocarcinoma | 6 (7.8%) |
Solid trabecular adenocarcinoma | 4 (5.1%) |
Total | 77 (100%) |
Note: abs - absolute value in percentage | |
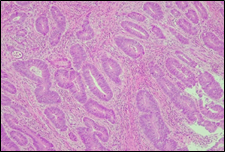
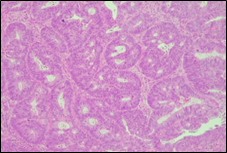
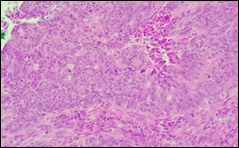
The present study concluded that, out of the total 77 patients with colorectal neoplasia, 17 (22.1%) had a high degree of differentiation (G1), 51 (66.2%) had a moderate degree of differentiation (G2), and 9 (11.7%) had a low degree of differentiation (G3). Figure 3 illustrates the different morphopathological forms of colorectal neoplasia observed in the patients included in the study.
A | B |
C | |
Fig. 3 Morphopathological forms of colorectal tumors (degree of differentiation) A – conventional highly differentiated colonic adenocarcinoma (G1) (HE stain, ×200) B – moderately differentiated (G2) conventional colonic adenocarcinoma (HE stain, ×200) C – conventional poorly differentiated colonic adenocarcinoma (G3) (HE stain, ×200) | |
For patients with colorectal neoplasm, investigating the correlation between the macroscopic aspect of the tumor formation visualized endoscopically and the degree of differentiation described histologically, it was found that there is no statistically significant relationship (p > 0.05), which is above the threshold accepted for demonstrating a significant statistical correlation. It is known that most colorectal adenocarcinomas develop at the site of precursor lesions, such as adenomas and dysplasia. Residual adenoma is a phenomenon commonly found in colorectal adenocarcinomas. Typical adenomas are subclassified into tubular, tubulovillous, and villous types based on their architectural and histological features. Tubular adenomas consist of dysplastic glands that resemble cryptic intestinal glands and contain less than 25% villous component. Villous adenomas are composed of more than 75% villous components, which appear as fibrovascular rods covered by dysplastic epithelium. Tubulovillous adenomas represent intermediate lesions with a villous component ranging from 25% to 75%.
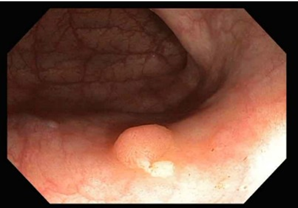
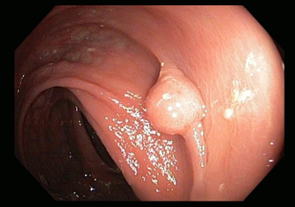
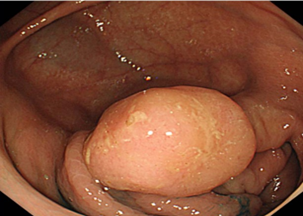
Premalignant lesions are described in terms of number, shape, size, location, histological type, and degree of malignancy. In terms of shape, the polyps were sessile, semipediculated or pedicled, round-oval, or polylobate (Figure 4).
B | |
C | D |
Fig. 4 Endoscopic appearance of polypsA – sessile polyp; B – semipediculated polyp; C – pedunculated polyp; D – voluminous villous polyp | |
Numerically, polyps can be solitary, multiple, or present in very large numbers, which can reach up to thousands (as in familial adenomatous polyposis, an autosomal dominant hereditary pathology). These can be detected by chance in a family member or transmitted through a genetic abnormality across multiple generations.
In the present research, regarding the number of polyps, solitary polyps were detected in 166 (93.2%) patients, two polyps in 10 (5.6%) patients, and three and more polyps in 2 (1.1%) patients.
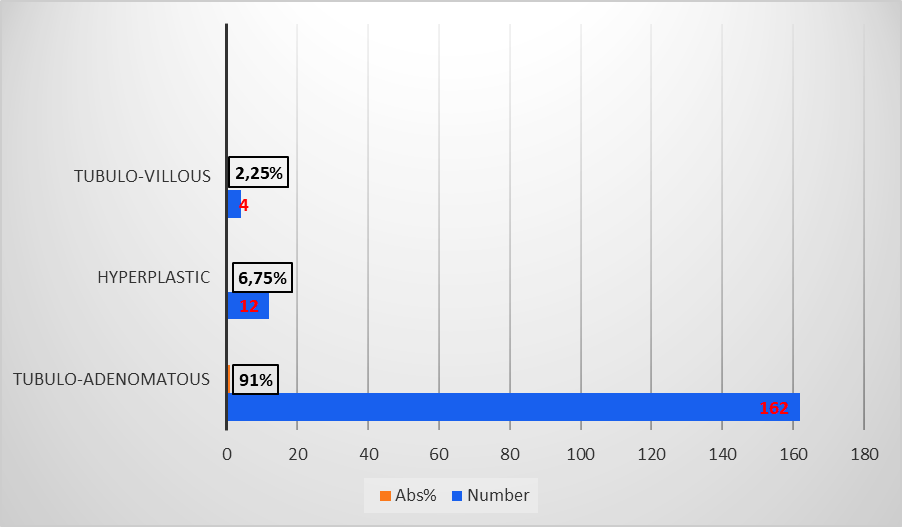
In terms of size, polyps were categorized as follows: under 5 mm (very small polyps), between 5 and 10 mm (small polyps), and over 10 mm (large polyps) [9]. Very small polyps predominated in 86 (48.3%) cases, small polyps in 61 (34.3%), and large polyps represented 17.4% (n = 31). Regarding the histological type, the majority were neoplastic, with tubuloadenomatous polyps predominating (162, 91%), followed by hyperplastic polyps (12, 6.75%) and tubulovillous polyps (4, 2.25%) (Figure 5).
|
Fig. 5 Histological types of premalignant colonic lesions in patients included in the study Note: abs - absolute value in percentage |
The distribution of polyps varied statistically significantly (p = 0.0001) in relation to polyp size and histology. The most frequent, tubuloadenomatous (n = 86), were of very small size, while tubulovillous polyps (n = 4) were of large size, and hyperplastic polyps (n = 12) were equally distributed between small and large sizes (Table 3).
Table 3. Associative distribution of polyps in relation to size and histological type | |||||
Size | Total | ||||
very small | small | large | |||
tubuloadenomatous | frequency % of histology | 86 (48.3%)* | 55 (30.9%) | 21 (11.8%)* | 162 (91%) |
tubulovillous | frequency % of histology | - | - | 4(2.2%) | 4 (2.2%) |
hyperplastic | frequency % of histology | - | 6 (3.4%) | 6 (3.4%) | 12 (6.8%) |
| Total/size | 86 (48.3%) | 61 (34.3%) | 31 (17.4%) | 178 (100%) |
Note: p < 0.05*, which allows us to conclude that very small sizes are much more frequent than large sizes | |||||
The results indicate that the distribution of case sizes is not uniform, and there is a significant difference between very small, small, and large sizes. Thus, p < 0.05 suggests that very small sizes are much more frequent than large sizes.
Dysplasia refers to a pathological adaptive cellular alteration, which involves changing in volume, shape, and organization within a tissue. This includes the variation in the size and shape of the cells, an increase in the volume of the nuclei (which become irregular and hyperchromic) disturbances in the stratification, disproportion between layers, dedifferentiation, depolarization of cells, and a disordered arrangement of cells within the epithelium. Depending on the severity of these changes, dysplasia can be classified as mild (simple), moderate, or severe. The World Health Organization (WHO) updated its classification system in 2019 to use a two-grade system: low-grade dysplasia (encompassing mild and moderate dysplasia) and high-grade dysplasia.
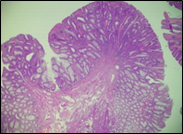
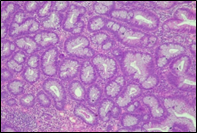
According to the degree of dysplasia, the premalignant lesions were described as follows: 106 (59.5%) with mild dysplasia, 48 (26.8%) with moderate dysplasia, and 24 (13.5%) with severe dysplasia. When grouped according to the WHO classification (2019), the premalignant lesions exhibited low-grade dysplasia in 154 (86.5%) patients and high-grade dysplasia in 24 (13.5%). Figure 6 shows different morphopathological forms of premalignant lesions from the series of patients included in the study.
A | B | C |
D | E | F |
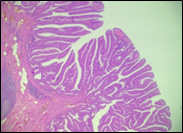
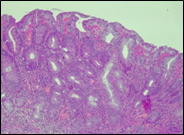
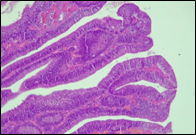
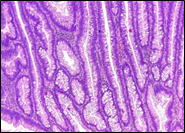
Fig. 6 Morphological subtypes of conventional adenomas according to the WHO classification (2019) A, B – tubular adenoma with moderate epithelial dysplasia (A - HE stain, ×100; B - HE stain ×200); C – villous adenoma with moderate epithelial dysplasia (HE stain, x100); D – tubular adenoma with high epithelial dysplasia (HE stain, x200); E – tubulovillous adenoma with high epithelial dysplasia (HE stain, x200); F – tubulovillous adenoma with low epithelial dysplasia (HE stain, x200) | ||
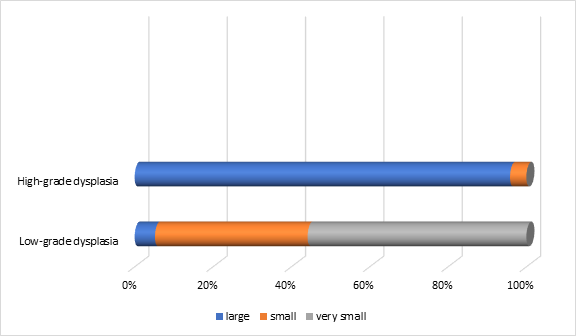
The distribution of polyps varied statistically significantly (p=0.0001) in relation to the size and dysplasia of the premalignant lesions. The smallest size was recorded in polyps with low-grade dysplasia (n=153), while large sizes were more commonly found in those with high-grade dysplasia (n=23). This demonstrates a direct relationship between size and dysplasia, with 74.2% of polyps with high dysplasia being classified as large (Figure 7).
|
Fig. 7 Distribution of polyps in relation to size and their degree of dysplasia in the patients included in the study Note: size of the polyps – large, small, very small |
Discussions
As cell proliferation exceeds degradation, accumulations of cells appear, forming small protrusions in the intestinal lumen that represent benign tumors (polyps). Adenomatous polyps are defined as lesions containing epithelial neoplasia. According to their histological structure, adenomas can be of three types. The most common are tubular adenomas, which are formed predominantly (more than 80%) from a complex network of branched adenomatous glands. The second type is represented by villous adenomas, composed predominantly (>75%) of adenomatous glands that extend linearly from the surface to the center of the polyp, creating a digitiform appearance. The third type of adenomatous polyp is represented by tubulovillous lesions, which are a combination of the other two histological types (25-75%) [10]. Colorectal adenomas are, by definition, considered dysplastic, as they all contain foci of dysplasia. At their level, the glandular epithelium exhibits abnormalities in cellular differentiation and renewal, leading to hypercellularity in the colonic crypts, with cells containing mucin in variable amounts. Based on the extent of cytological and architectural alterations, two types of dysplasia are described: mild and severe [11, 12].
Currently, the architecture of the colonic mucosa is also considered in determining the form of neoplastic growth. According to the Paris classification, colonic neoplastic lesions are categorized into the following types:
type Ip – protruding, pedunculated
type Is – protruding, sessile, broad-based
type IIa – superficial, flat, and elevated
type IIb – completely flat
type IIc – superficially depressed
type III – excavated/ulcerated [13].
Kudo classifies adenomas into protruding (polypoid lesions), flat-elevated, and flat (Figure 8). Flat-elevated lesions are further divided into flat-elevated and flat-elevated with central depression. The flat type of lesion is subdivided into flat and depressed. In this classification, the flat type corresponds to the initial description of flat adenomas. within 2001, Kudo and colleagues morphologically analyzed approximately 20,000 colonoscopic lesions and determined that polypoid and non-polypoid lesions accounted for 55% and 45% of cases, respectively. Non-polypoid lesions are present in the proximal and distal regions of the colon in equal proportion, whereas polypoid lesions are more frequent in the distal colon [14, 15].
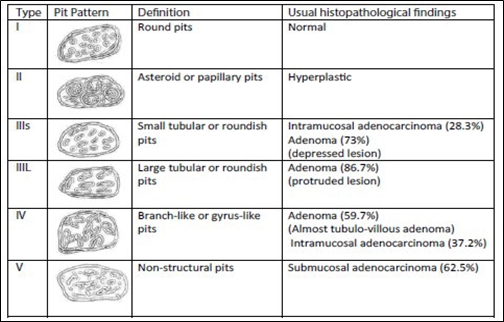 |
Fig. 8 Kudo classification of colonic neoplastic lesions [14, 15] Note: Pit pattern classification of colorectal neoplasia (Kudo et al.). I – Round pit (normal pit), II – asteroid pit, IIIS – tubular or round pit (smaller than the normal pit, i.e., type I), IIIL – tubular or round pit (larger than the normal pit, i.e., type I), IV – dendritic or gyrus-like pit, V – amorphous, nonstructured pit. |
World Health Organization (WHO) in 2010 classified serrated polyps into non-dysplastic polyps, which include hyperplastic polyps and sessile serrated polyps, and dysplastic polyps, which include sessile serrated polyps with dysplasia and traditional serrated adenomas [16, 17].
The prognostic significance of histopathological grading has been recognized in numerous studies over time. In 1949, Dukes found a correlation between colonic tumor grading and lymph node metastasis, indicating a worse prognosis. Poorly differentiated adenocarcinomas are associated with lymph node metastases in more than 50% of cases. In contrast, moderately and highly differentiated tumors have a lower rate of lymph node metastasis. The degree of tumor differentiation also has prognostic significance, as it correlates with local invasion of the intestinal wall and adjacent organs, as well as with venous and lymphatic invasion. An obvious relationship between survival rate and histopathological grading of colorectal neoplasia has been demonstrated. The higher the grading–meaning the more undifferentiated the tumor–the more aggressive its progression and the lower the chances of long-term survival. According to Morson (2001), the five-year survival rates for highly differentiated, moderately differentiated, and undifferentiated forms are 80%, 60%, and 25%, respectively [18, 19].
However, there are challenges in interpreting studies due to the use of various older grading systems. Additionally, classifying tumors according to the current staging system, which includes 4 grades–from G1 (well-differentiated) to G4 (undifferentiated, anaplastic) – can be complex. There may also be staging errors, as most invasive tumors contain focal areas of undifferentiated cells at the site of invasion. However, these areas are not necessarily representative of the entire tumor’s grade [20, 21].
The various histopathological types of primary colonic neoplasms may allow for the classification of patients into different prognostic groups. The histopathological aspect has negative prognostic significance that has been proven only for certain histological subtypes of adenocarcinomas: colloid (mucinous) carcinoma, "signet ring" cell carcinoma, small cell carcinoma, and squamous carcinoma. Despite advances in surgery and adjuvant therapy for colonic neoplasms in recent years, the average 5-year overall survival of patients with curative resections for CRC remains only 62%, and local recurrences occur in more than 90% of cases where therapy has failed [22]. The prognosis of CRC depends on a multitude of factors that can be grouped into categories: clinical factors, tumor stage factors, histopathological factors, and biological (molecular) factors [23]. Undoubtedly, the most important factors for survival prognosis are the degree of tumor invasion into the intestinal wall. Obviously, the more advanced the stage of neoplasia, the shorter the life expectancy. The TNM classification for CRC is based on the observation that the size of the tumor correlates directly with local invasion, and thus, implicitly with the prognosis. No significant relationship between neoplasm size or diameter and 5- or 10-year survival has been demonstrated. Moreover, some studies suggest that large-sized NPCs may have a better prognosis. A possible explanation for this could be that the size of the tumor is often amplified by peritumoral inflammatory phenomena of the body's defense mechanisms, while in reality, the actual tumor volume is smaller.
The macroscopic anatomopathological form is a parameter in formulating the prognosis, as the appearance of the neoplasia reflects its biological nature. Patients with exophytic or polypoid tumors seem to have a better prognosis compared to those with ulcerative or infiltrative tumors. Grinnell, who classifies tumors into protruding (exophytic, vegetative), intermediate, and infiltrative types, found as early as 1939 that 83% of patients with vegetative neoplasms survive five years, while the percentage drops to only 38% for those with infiltrative tumors or 48% for those with intermediate tumors. The explanation for these differences lies in the lower percentage of intestinal wall penetration in vegetative tumors compared to ulcerative ones (24% vs 39%), the lower frequency of nodal and/or hematogenous metastasis in vegetative tumors (about 24% vs 31 % for infiltrative tumors), and the fact that, in general, vegetative tumors are more limited in depth within the intestinal wall compared to ulcerative-infiltrative ones [24, 25]. There are divergent opinions on how the location of CRC in various segments of the colon influences survival. One study suggests that the location of CRC in the subperitoneal space on the rectum decreases the 5-year survival rate compared to intraperitoneal locations. Tumors located in the right hemicolon tend to have a less favorable prognosis than those in the left hemicolon, with a survival rate 2-14% higher at 3 years post-resection for neoplasms in the left hemicolon compared to those on the right. This difference is probably explained by the more technically challenged dissection of the central lymphatic stations at the level of the origin of the superior mesenteric artery [26]. The histopathological aspect of the primary neoplasm has negative prognostic significance proven only in the case of certain histological subtypes of adenocarcinomas: colloid (mucinous) carcinoma, "signet ring " cell carcinoma, small cell carcinoma, and squamous carcinoma. These adenocarcinomas are aggressive tumors, usually discovered at a more advanced stage, with extensive pericolonic invasion. They present an increased incidence of lymph node metastases and have a high degree of malignancy, leading to lower long-term survival rates [27]. The effects of the main histopathological prognostic factors in CRC and NPC are shown in Table 4.
Table 4. Histopathological prognostic factors in CRC and NPC [20-27] | |
Histopathological prognostic factors | Effect on prognosis |
TNM stage (documented histopathologically) | increasing the stage significantly decreases the prognosis |
The degree of intestinal wall invasion | the depth of invasion negatively affects the prognosis |
The degree of differentiation | well-differentiated tumors have a better prognosis than poorly- or undifferentiated ones |
Local inflammatory and immunological reaction | favorable prognosis |
Primary tumor morphology | polypoid/exophytic tumors have a better prognosis than ulcerative/infiltrative ones |
The size of the primary tumor | no effect in most studies |
Tumor location in the colon | better prognosis for colon tumors compared to rectal tumors * better prognosis for left hemicolon locations compared to right hemicolon * |
Presence of lymphovascular invasion (LVI) and perineural invasion (PNI) | favorable prognosis in their absence |
Status of surgical resection margins | favorable for negative resection margins |
Status of lymph nodes (total number identified, number of positive lymph nodes) | favorable for the lack of metastases in the lymph nodes |
Note: * - prognostic significance for which the conducted studies are contradictory | |
Research on the molecular biology of CRC, along with increasingly detailed genomic studies, raises hope for better control of this pathology. The only chance to improve patient survival is through early diagnosis, appropriate management at each stage by a multidisciplinary team, and active, systematic surveillance of all patients. Treatment must be differentiated by stage and tailored to each patient, who may develop a unique form of neoplasia. Stage 0 malignant polyps (TisN0M0) less than 2 cm, with submucosal infiltration, endoscopically resected, with non-infiltrating margins, well-differentiated cancer in situ, or severe dysplasia, which usually have a low chance of lymph node infiltration, can be followed up colonoscopically. If the pedunculated polyps are over 2 cm or the microscopic malignant tissue is poorly differentiated, or if the invasion exceeds the submucosa or there is suspicion of damage to a single lymph node, segmental colectomy with lymphadenectomy is required after polypectomy. In cases where endoscopic resection margins are positive, immediate colectomy is mandatory [28]. In stages I and II (any T, N0, M0), where the neoplasia is considered localized, surgery is the standard treatment. For T3 tumors, a D3 lymphadenectomy is required, involving complete dissection of all regional, paracolic, intermediate, and central lymph nodes (a minimum of 12 lymph nodes should be removed and examined). In stage III, where lymph node invasion is demonstrated (any TN1-3M0), if there is a risk of local or distant recurrence, sectoral colectomy with D3 lymphadenectomy is necessary. This procedure is important for correct stagingand should be followed by adjuvant chemotherapy [29].
Active surveillance of patients who have undergone surgery for CRC, as well as those at an increased risk of developing this neoplasia (e.g., patients who have undergone polypectomies for stage 0 cancer), is essential. The most effective way to detect recurrences and, especially, metastases in a timely manner is the systematic follow-up of patients with CRC in stages I-III. The most comprehensive surveillance protocol is that recommended by the Japanese Colorectal Cancer Society (2019), which recommends:
clinical examination and determination of CEA (Carcinoembryonic Antigen) every 3 months during the first 3 years and every 6 months up to 5 years.
abdominal ultrasound and chest X-ray every 6 months for up to 5 years.
thoraco-abdominal computed tomography and colonoscopy annually for up to 5 years [30].
The surgeon, as a member of the multidisciplinary team managing colorectal neoplasia, plays an important role in diagnosis, treatment, and follow-up.
Conclusions
A detailed analysis of the morphopathological characteristics of tumor formations, beyond confirming malignancy, provides important information for determining therapeutic management. Classification into a risk group that assigns the patient a specific prognosis can also contribute to a better understanding of tumor genesis, allowing for the stratification of patients for individualized treatment–either more aggressive or less aggressive–based on the prognosis. Further extensive studies are required to analyze the correlations between histopathological form, treatment, and survival rate.
Competing interests
None declared.
Authors’ contributions
AU – conceptualization, investigation, methodology, writing – original draft, writing – review and editing, visualization, project administration, data curation, resources. GR – investigation, project administration, validation, supervision, data curation. AD – data acquisition. MC, EM – analysis and interpretation of data. All authors critically reviewed the work and approved the final version of the manuscript.
Acknowledgment
No external funding.
Patient consent
Obtained.
Ethics approval
Favorable opinion of the Research Ethics Committee of the Nicolae Testemițanu State University of Medicine and Pharmacy, No. 52 dated 16.03.2018 and No. 7 dated 18.05.2022.
Provenance and peer review
Not commissioned, externally peer reviewed.
Authors’ ORCID IDs
Alexandr Ursu – https://orcid.org/0000-0002-2753-418X
Andrei Dolghii – https://orcid.org/0009-0001-4660-2440
Mihaela Cozma – https://orcid.org/0000-0001-9405-4437
Eugen Melnic – https://orcid.org/0009-0000-9222-544X
Gheorghe Rojnoveanu – https://orcid.org/0000-0001-7075-4113
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424. doi: 10.3322/caac.21492.
Bujanda L, Cosme A, Gil I, Arenas-Mirave I. Malignant colorectal polyps. World J Gastroenterol. 2010;16(25):3103-3111. doi: 10.3748/wjg.v16.i25.3103.
Târcoveanu E. Cancerul de colon – o problemă de sănătate publică [Colon cancer – a public health problem]. [J Surg] (Iași). 2007;3(4):34-41. Romanian.
Japanese Research Society for Cancer of the Colon and Rectum. General rules for clinical and pathological studies on cancer of the colon, rectum and anus. Jpn J Surg.1983;13(6):557-573. doi: 10.1007/BF02469505.
Nikolaou S, Qiu S, Fiorentino F, Rasheed S, Tekkis P, Kontovounisios C. Systematic review of blood diagnostic markers in colorectal cancer. Tech Coloproctol. 2018;22(7):481-498. doi: 10.1007/s10151-018-1820-3.
Meng C, Yin X, Liu J, Tang K, Tang H, Liao J. TIMP-1 is a novel serum biomarker for the diagnosis of colorectal cancer: a meta-analysis. PLoS One. 2018;13(11):e0207039. doi: 10.1371/journal.pone.0207039.
Ahlquist D, Zou H, Domanico M, Mahoney DW, Yab TC, Taylor WR, et al. Next-generation stool DNA test accurately detects colorectal cancer and large adenomas. Gastroenterology. 2012;142(2):248-56. doi: 10.1053/j.gastro.2011.10.031.
Pisano M, Zorcolo L, Merli C, et al. 2017 WSES guidelines on colon and rectal cancer emergencies: obstruction and perforation. World J Emerg Surg. 2018;13:36. doi: 10.1186/s13017-018-0192-3.
Kumar V, Abbas AK, Aster J, editors. Robbins and Cotran pathological basis of disease. Philadelphia: Elsevier/Saunders; 2015. 1391 p.
Itzkowitz S, Potack J. Colonic polyps and polyposis syndromes. In: Feldman M, Friedman LS, Brandt LJ, editors. Sleisenger and Fordtran's gastrointestinal and liver disease: pathophysiology, diagnosis, management. 9th ed. Philadelphia: Saunders/Elsevier; 2010. p. 31-44.
Robert M. The malignant colon polyp: diagnosis and therapeutic recommendations. Clin Gastroenterol Hepatol. 2007;5(6):662-667.doi: 10.1016/j.cgh.2007.04.001.
Sweetser S, Smyrk T, Sugumar A. Serrated polyps: critical precursors to colorectal cancer. Expert Rev Gastroenterol Hepatol. 2011;5(5):627-635. doi: 10.1586/egh.11.67.
The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon. Gastrointest Endosc. 2003;58(6 Suppl):S3-43. doi: 10.1016/s0016-5107(03)02159-x.
Kudo S, Kashida H, Tamura T, et al. Colonoscopic diagnosis and management of nonpolypoid early colorectal cancer. Word J Surg. 2000;24(9):1081-1090.doi: 10.1007/s002680010154.
Rembacken B, Fujii T, Cairns A, et al. Flat and depressed colonic neoplasms: a prospective study of 1000 colonoscopies in the UK. Lancet. 2000;355(9211):1211-1214. doi: 10.1016/s0140-6736(00)02086-9.
Snover D, Ahnen D, Burt R, et al. Serrated polyps of the colon and rectum and serrated polyposis. In: Nagtegaal ID, Arends MJ, Odze RD, Lam AK. WHO Classification of Tumours of the Digestive System. Lyon: IARC Press; 2010. p. 160-165.
Sobin LH, Gospodarowicz MK, Wittekind C, editors; Union for International Cancer Control UICC. TNM Classification of Malignant Tumors. 7th ed. Hoboken: John Wiley & Sons; 2009. p. 100-105.
Morino M, Allaix ME, Caldart M, et al. Risk factors for recurrence after transanal endoscopic microsurgery for rectal malignant neoplasm. Surg Endosc. 2011;25(11):3683-3690. doi: 10.1007/s00464-011-1777-z.
Friel C, Cromwell JW, Marra C, et al. Salvage radical surgery after failed local excision for early rectal cancer. Dis Colon Rectum. 2002;45(7):875-879. doi: 10.1007/s10350-004-6320-z.
Day LW, Velayos F. Colorectal cancer screening and surveillance in the elderly: updates and controversies. Gut Liver. 2015;9(2):143-151. doi: 10.5009/gnl14302.
Lu J, Lin GL, Qiu HZ, et al. Comparison of transanal endoscopic microsurgery and total mesorectal excision in the treatment of T1 rectal cancer: a meta-analysis. PLoS One. 2015;10(10):14-27. doi: 10.1371/journal.pone.0141427.
Zauber AG, Winawer S, O'Brien J. Colonoscopic polypectomy and long-term prevention of colorectal cancer deaths. N Engl J Med. 2012;366(8):687-696.doi: 10.1056/NEJMoa1100370.
Niv Y, Half N, Moshkowitz M, et al. Surveillance of patients after colonoscopic polypectomy and curative resection of colorectal cancer. Harefuah. 2010;149(10):670-673.
Mills SE, editor. Histology for pathologists. Philadelphia: Wolters Kluwer; 2012. p. 316-322.
Grigorescu M, Irimie A, Beuran M, editors. Tratat de oncologie digestivă [Treatise on digestive oncology]. Vol. 3. Bucharest: Editura Academiei Române; 2015. p. 205-227. Romanian.
Bărbulescu M. Factorii prognostici ai cancerului colorectal [Prognostic factors of colorectal cancer]. [J Surg] (Iași). 2007;3(2):117-129. Romanian.
Osorio H, Finol HJ, Gonzalez LR, et al. Ultrastructure of colorectal adenocarcinoma and peritumoral tissue in untreated patients. Ultrastruct Pathol. 2018;42(2):81-90. doi: 10.1080/01913123.2017.1422064.
Voloyiannis T, Snyder M, Bailey R, Pidala M. Management of the difficult colon polyp referred for resection. Dis Colon Rectum. 2008;51(3):292-295.doi: 10.1007/s10350-007-9175-2.
Tholoor S, Tsagkournis O, Basford P, Bhandari P. Managing difficult polyps: techniques and pitfalls. Ann Gastroenterol. 2013;26(2):114-121.
Hashiguchi Y, Muro K, Saito Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol. 2020;25(1):1-42. doi: 10.1007/s10147-019-01485-z.